CBSE Class 12 exams will be conducted from April 26 to June 15, 2022 for Term 2. You can practice questions here daily.
CBSE has released CBSE Sample Paper Class 12 on its official website along with the marking scheme for Term 2 examinations. Students must practicse from them daily to assss their preparations and thereafter focus on weaker areas.
Also Read: CBSE Term 2 exam guidelines released for classes 10 and 12
Shiksha.com brings to you daily practice questions that will help you in better preparation of the exams. This blog is a compilation of subject questions from the CBSE Sample paper Class 12 2022. Get started with the CBSE practice test by solving the CBSE question bank below.
LIVE UPDATE
- 5:39 PM IST• 13 May 2022
CBSE Class 12 Biology Syllabus
Unit
Chapters
Marks
Unit - VIII
Biology and Human Welfare
Human Health and Disease
14
Microbes in Human Welfare
Unit IX - Biotechnology and Its Applications
Biotechnology: Principles and Processes
11
Biotechnology and its Applications
Unit X - Ecology and Environment
Organisms And Population
10
Biodiversity and its Conservation
- 4:05 PM IST• 12 May 2022
CBSE Class 12 Physics Syllabus Term 2
Unit
Chapters
Marks
Unit – V
Electromagnetic Waves
17
Chapter–8: Electromagnetic Waves
Unit – VI
Optics
Chapter–9: Ray Optics and Optical Instruments
Chapter–10: Wave Optics
Unit - VII
Dual Nature of Radiation and Matter
11
Chapter–11: Dual Nature of Radiation and Matter
Unit–VIII
Atoms and Nuclei
Chapter–12: Atoms
Chapter–13: Nuclei
Unit IX
Electronic Devices
7
Chapter–14: Semiconductor -Electronics: Materials, Devices and Simple Circuits
Total
35
- 4:09 PM IST• 11 May 2022
CBSE Class 12 Sample Questions Biology- May 11
Check answer here
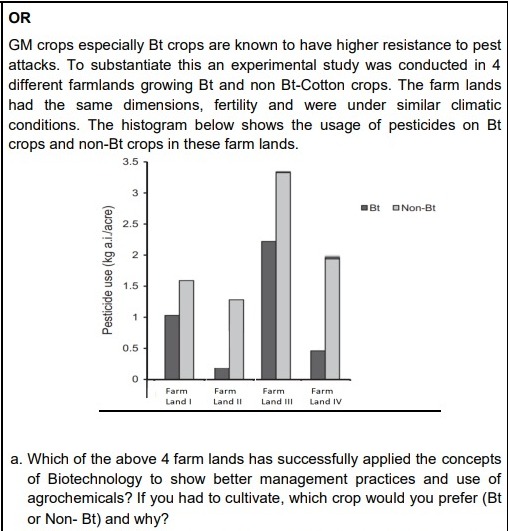
(b) In Bt cotton a cry gene has been introduce from bacterium Bacillus thuringiensis (Bt) which causes synthesis of a toxic This protein becomes active in the alkaline gut of bollworm feeding on cotton, punching holes in the lining causing death of the insect. However; a Non Bt crop will have no effect on the cotton bollworm/ the yield of cotton will decrease / non Bt will succumb to pest attack. - 2:06 PM IST• 10 May 2022
CBSE Class 12 Sample Questions Physics - May 10
- 6:35 PM IST• 6 May 2022
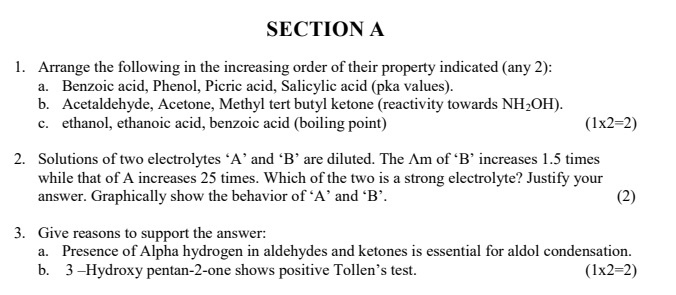
CBSE Class 12 Sample Questions Chemistry- May 6
- 6:06 PM IST• 6 May 2022
CBSE Class 12 Sample Questions Chemistry- May 6
- 5:07 PM IST• 6 May 2022
CBSE Class 12 Sample Questions Chemistry- May 6
Check answers here
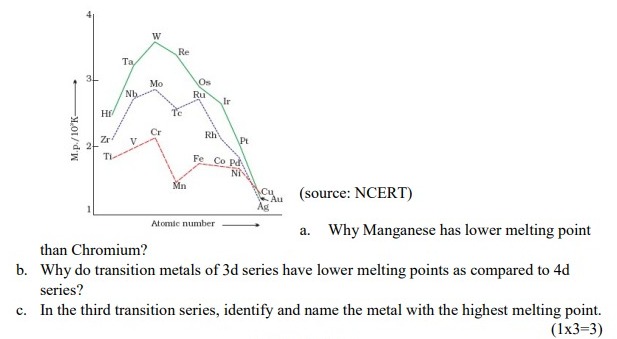
(a) Manganese is having lower melting point as compared to chromium , as it has highest number of unpaired electrons , strong interatomic metal bonding , hence no delocalisation of electrons . (b) There is much more frequent metal – metal bonding in compounds of the heavy transition metals i.e 4d and 5d series , whixh accounts for lower melting point of 3d series. (c) Tungsten - 2:16 PM IST• 6 May 2022
CBSE Class 12 Sample Questions Chemistry- May 6
Check answers here
(a) The ability of fluorine to stabilize the highest oxidation state is attributed to the higher lattice energy or high bond enthalpy. (b) Co2+ has three unpaired electrons so it would be paramagnetic in nature, hence Co2+ ion would be attracted to magnetic field. (c) The transition elements of 5d series have intervening 4f There is greater effective nuclear charge acting on outer valence electrons due to the weak shielding by 4f electrons. Hence first ionisation energy of 5 d series of transition elements are higher than that of 3d and 4d series. - 7:31 PM IST• 5 May 2022
CBSE Class 12 Sample Questions Chemistry- May 5
Check answer here
Al(s) /Cd2+ (0.1M) // Al3+ (0.01M) /Cd(s) 2Al(s) + 3Cd2+ (0.1M) à 3Cd (s) + 2Al3+ (0.01M) Ecell = Eocell -0.059/n log [Al3+]2 n [Cd2+]3 Ecell= 1.26 – .059/6 log (0.01)2 (0.1)3 = 1.26 – 0.059 (-1) 6 = 1.26+0.009 = 1.269 V - 12:08 PM IST• 5 May 2022
CBSE Class 12 Sample Questions Physics - May 5
- 7:41 PM IST• 4 May 2022
CBSE Class 12 Sample Questions Biology- May 4
Check answer here
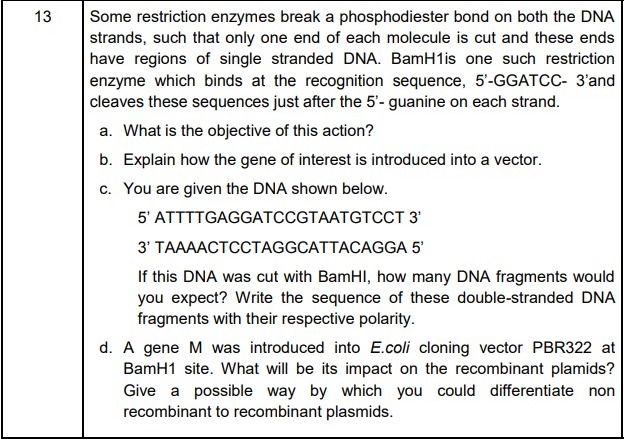
13. (a) The two different DNA molecules will have compatible ends to recombine. - 6:30 PM IST• 4 May 2022
CBSE Class 12 Sample Questions Chemistry- May 4
Check the answers here
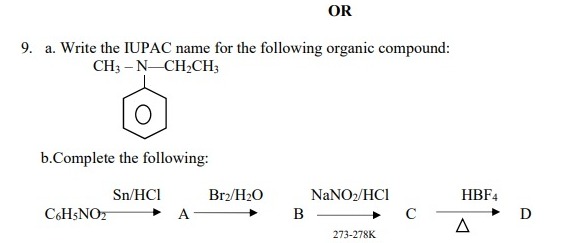
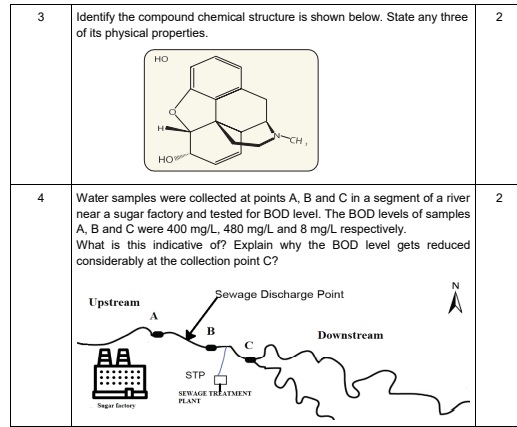
(a). N-Ethyl-N-methylbenzenamine or N-Ethyl-N-ethylaniline (b). Refer to the official website of CBSE for the diagram based answer - 4:07 PM IST• 4 May 2022
CBSE Class 12 Sample Questions Physics - May 4
Check the answers here
(a). The total intensity at a point where the phase difference is ?, is given by ???? = ????1 + ????2 + 2?????1????2 ???????????? ?. Here ????1 and ????2 are the intensities of two individual sources which are equal. When ? is 0, I = 4????1. When ? is 90o, I = 0 Thus intensity on the screen varies between 4????1 and 0. - 5:22 PM IST• 2 May 2022
CBSE Class 12 Sample Questions Biology- May 2
Check answers here
(a) The two different DNA molecules will have compatible ends to (½ mark) (b) Restriction enzyme cuts the DNA of the vector and then ligates the gene of interest into the DNA of the (1 mark) (c) 2 fragments (½ mark) 5’ ATTTTGAG 3’5’GATCCGTAATGTCCT 3’ 3’ TAAAACTCCTAG 5’.3’GCATTACAGGA 5’ (1 mark) (d) BamH1 site will affect tetracycline antibiotic resistance gene, hence the recombinant plasmids will lose tetracycline resistance due to inactivation of the resistance gene. (1 mark) Recombinants can be selected from non recombinants by plating into a medium containing tetracycline, as the recombinants will not grow in the medium because the tetracycline resistance gene is cut. (1 mark) - 4:49 PM IST• 2 May 2022
CBSE Class 12 Sample Questions Chemistry- May 2
Check answers here
9. (a) When N-ethylethanamine reacts with benzenesulphonyl chloride, N,N-diethylbenzenesulphonamide is (b) When benzylchloride is treated with ammonia, Benzylamine is formed which on reaction with Chloromethane yields a secondary amine, N-methylbenzylamine. (c) When aniline reacts with chloroform in the presence of alcoholic potassium hydroxide, phenyl isocyanides or phenyl isonitrile is formed. - 12:45 PM IST• 2 May 2022
CBSE Class 12 Sample Questions Physics - May 2
Check answers here
(a). If the Earth did not have atmosphere, then there would be absence of greenhouse effect of the atmosphere. Due to this reason, the temperature of the earth would be lower than what it is now. (b). An e.m. wave carries momentum with itself and given by P = Energy of wave(U)/ Speed of the wave(c) = U/c when it is incident upon a surface it exerts pressure on it. - 4:58 PM IST• 29 Apr 2022
CBSE Class 12 Sample Questions Biology - April 29
Check answers here
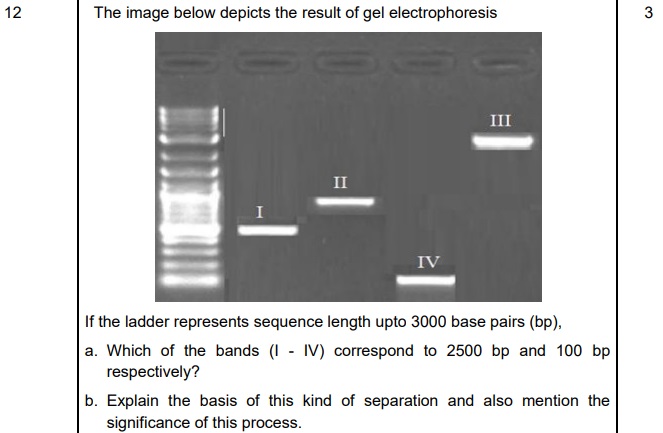
A. a. Band III corresponds to 2500 base pairs, and Band IV corresponds to 100bp. (½ + ½ mark)
b. The fragments will resolve according to their size. The shorter sequence fragments would move farthest from well as seen in Band IV (100 bp) which is lighter as compared to Band III which is heavier being 2500 base pairs. (1 mark)
The significance of electrophoresis is to purify the DNA fragments for use in constructing recombinant DNA by joining them with cloning vectors. - 3:42 PM IST• 29 Apr 2022
CBSE Class 12 Sample Questions Chemistry - April 29
Check answers here
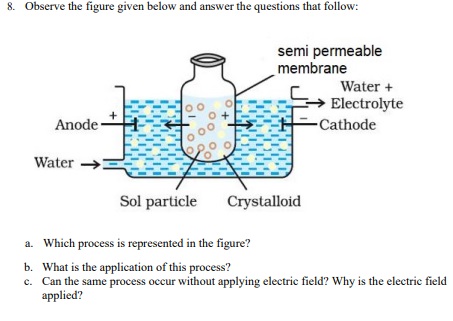
A. (a)electrodialysis
(b) purification of colloidal solution
(c) Yes. Dialysis is a very slow process to increase its speed electric field is applied - 3:38 PM IST• 29 Apr 2022
CBSE Class 12 Sample Questions Physics - April 29
Check answer here
A. Microwaves are suitable for the radar system used in aircraft navigation. Range of frequency of microwaves is 108 Hz to 1011 Hz. - 4:03 PM IST• 28 Apr 2022
CBSE Class 12 Sample Questions Biology - April 28
- 2:28 PM IST• 28 Apr 2022
CBSE Class 12 Sample Questions Chemistry - April 28
- 12:11 PM IST• 28 Apr 2022
CBSE Class 12 Sample Questions Physics - April 28
- 3:40 PM IST• 27 Apr 2022
CBSE Class 12 Sample Questions Biology - April 27
- 3:06 PM IST• 27 Apr 2022
CBSE Class 12 Sample Questions Chemistry - April 27
- 1:20 PM IST• 27 Apr 2022
CBSE Class 12 Sample Questions Physics - April 27
- 6:38 PM IST• 26 Apr 2022
CBSE Class 12 Sample Questions Biology - April 26
- 4:29 PM IST• 26 Apr 2022
CBSE Class 12 Sample Questions Chemistry - April 26
- 12:50 PM IST• 26 Apr 2022
CBSE Class 12 Sample Questions Physics - April 26
- 12:04 PM IST• 25 Apr 2022
CBSE Class 12 Sample Questions Biology - April 25
- 1:02 AM IST• 25 Apr 2022
CBSE Class 12 Sample Questions Chemistry - April 25
- 12:57 AM IST• 25 Apr 2022
CBSE Class 12 Sample Questions Physics - April 25
Check answers here
A. 6. Number of atoms present in 2 g of deuterium = 6 × 1023
Number of atoms present in 2.0 Kg of deuterium = 6 × 1026
Energy released in fusion of 2 deuterium atoms = 3.27 MeV
Energy released in fusion of 2.0 Kg of deuterium atoms
= 3.27/2 × 6 × 1026 MeV
= 9.81 × 1026 MeV
= 15.696 × 1013 J
Energy consumed by bulb per sec = 100 J
Time for which bulb will glow = 15.696 × 1013 /100
s = 4.97 × 104 year
7. A locus of points, which oscillate in phase is called a wavefront.
OR
A wavefront is defined as a surface of constant phase. - 4:14 PM IST• 22 Apr 2022
CBSE Class 12 Sample Questions Biology - April 22
- 2:27 PM IST• 22 Apr 2022
CBSE Class 12 Sample Questions Chemistry - April 22
- 1:54 PM IST• 22 Apr 2022
CBSE Class 12 Sample Questions Physics - April 22
- 12:03 PM IST• 22 Apr 2022
CBSE Class 12 Sample Questions Biology - April 22
- 6:08 PM IST• 21 Apr 2022
CBSE Class 12 Sample Questions Chemistry - April 21
5:40 PM IST• 21 Apr 2022CBSE Class 12 Sample Questions Physics - April 21
Follow Shiksha.com for latest education news in detail on Exam Results, Dates, Admit Cards, & Schedules, Colleges & Universities news related to Admissions & Courses, Board exams, Scholarships, Careers, Education Events, New education policies & Regulations.
To get in touch with Shiksha news team, please write to us at news@shiksha.com
Latest News
Next Story























Comments
(1)
M
2 years ago
Report Abuse
Reply to Manpreet singh