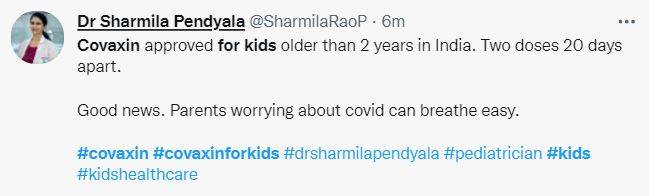Amid the ongoing reopening of schools across the nation, the approval of the vaccine has come as a sign of relief for many parents.
COVID-19 vaccine for kids! Bharat Biotech's Covaxin has become the first vaccine to be approved for kids. The vaccine has received emergency use approval from the Subject Expert Committee on COVID-19 for children in the 2-18 years age group. Amid the ongoing reopening of schools across the nation, the approval of the vaccine has come as a sign of relief for many parents. While talking to shiksha.com, Yamini Bharadwaj, whose children study in Delhi’s Abhinav Public School said, “If the news is true and the vaccine is safe for children then it is definitely good news. We all are worried about school reopening because the corona is still out there and we cannot trust children if they are following protocols or not. I will not be in school to see if my kids are using hand sanitizers or not. But if my kids will be vaccinated I will be tension-free.”
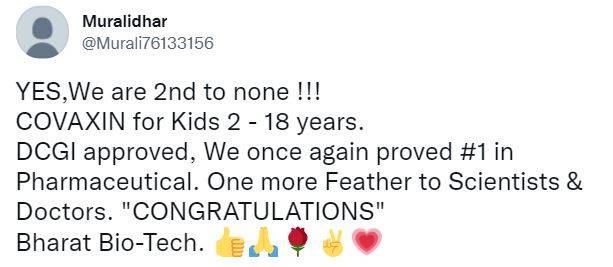
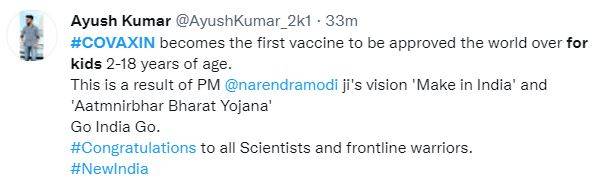
In September, Hyderabad-based Bharat Biotech’s Covaxin completed Phase-2 and Phase-3 trials on children below 18 years of age and submitted the trial data to the Drugs and Comptroller General of India (DCGI) at the start of this month. According to a media report, the subject expert panel said in a statement, “After detailed deliberation, the committee recommended for grant of market authorization of the vaccine for the age group of 2 to 18 years for restricted use in an emergency situation.” Just like for adults, for children as well, the made-in-India vaccine will be administered in two doses, with a gap of 20 days between the first and second dose.
Tweeple’s reaction to Covaxin’s approval for Kids
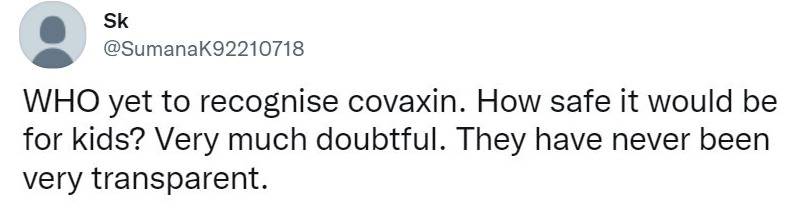
Meanwhile, there are people who are concerned if the use of the Covaxin Vaccine for Children is safe!
Reports suggest that the emergency use authorization received by the Subject Expert Committee for Covaxin is subject to certain conditions. The developer of Covaxin will continue the study as per Whole Virion, Inactivated Corona Virus Vaccine the approved clinical trial protocol. Prescribing information/Package Insert (PI), Summary of Product Characteristics (SmPC) and factsheet will also be required to be provided. Meanwhile, the WHO is yet to grant emergency use authorisation to Covaxin.
Follow Shiksha.com for latest education news in detail on Exam Results, Dates, Admit Cards, & Schedules, Colleges & Universities news related to Admissions & Courses, Board exams, Scholarships, Careers, Education Events, New education policies & Regulations.
To get in touch with Shiksha news team, please write to us at news@shiksha.com

"Writing is not about accurate grammar, it's about the honest thoughts you put in it". Having a versatile writing style, Anum loves to express her views and opinion on different topics such as education, entertainme... Read Full Bio