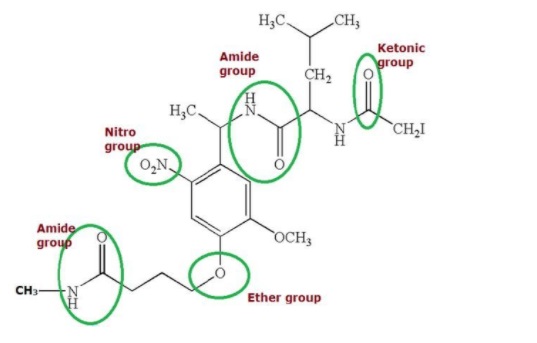
- What are Functional Groups?
- Role of Functional Groups
- FAQs regarding Functional Groups
What are Functional Groups?
In natural science, utilitarian groups are the substituent iotas or gatherings of particles that are appended to explicit atoms. These moieties (the piece of the atom which can be found in numerous different particles, too) are liable for the compound responses that the atom they are appended to partake in.
Role of Functional Groups
The way the utilitarian gatherings enjoy a synthetic response can be adjusted with other useful gatherings, and these gatherings can likewise be interconverted.
- Covalent holding joins the particles of these gatherings and the gathering overall to the atom
- On account of polymers, the utilitarian gatherings are by and large connected to the nonpolar centre of the carbon iotas in each rehashing unit of the related polymer, mixing the carbon chain with explicit compound attributes
- Some utilitarian gatherings have an ionic charge on them, as seen in carboxylate salts containing the - COO–ionic gathering
- When these gatherings are appended to atoms, they convert the particle into either compositions or polyatomic particles
- In a coordination complex, the focal's useful gathering is supposed to be a ligand.
Some more utilitarian gatherings containing components, for example, nitrogen and oxygen, including various hybridisations of the carbon-nitrogen and the carbon-oxygen securities, are shown underneath.
The presence of practical gatherings in an atom likewise influences the dissolvability and the propensity to frame buildings of the particle being referred to. If the utilitarian gatherings of the solute and the dissolvable associate well, the dissolvability increments. For instance, since sugar and water contain the - OH (hydroxyl) gathering, sugar can be effortlessly disintegrated in water.
In Class 12: In the chapter Alcohols, Phenols, and Ethers, functional groups have one of the major topics where their properties and groups are explained briefly. The chapter has a weightage of 28 Marks.
Explore exams which ask questions on Chemistry Alcohols, Phenols and Ethers
Select your preferred stream
FAQs regarding Functional Groups
Q. Through which descriptions, we can identify the functional group?
A. The gatherings of iotas, which are bound to the carbon spine of natural atoms, are useful. The mark substance responses of natural mixes are answerable for useful groupings.
Q. How can we define a carboxyl group?
A. A gathering of carboxyls is an ordinary gathering of capacities that appeared in science. A gathering of carboxyls is named having a gathering of carbonyls and hydroxyls, all bound to a carbon particle.
Q. List the functional groups.
A. A portion of the fundamental practical gatherings incorporates hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl gatherings in natural particles. These gatherings assume a huge part in framing atoms, for example, DNA, proteins, starches, and lipids.
Q. Can we say that alkyl is alcohol?
A. An aliphatic liquor in which a hydroxy gathering subs the aliphatic alkane chain is undefined.
Q. What do you mean by R in functional groups?
A. R gathering: A shortening for any gathering in which a carbon or hydrogen particle is joined to the remainder of the atom.
Q. Which is the major functional group?
Most elevated Priority Groups: Carboxylic Acids, Sulfonic Acids, Esters, Acid Halides, Amides. The "position rules" proceed in the accompanying request.
Q. Between OH and COOH, which is the highest in the functional group?
A. Oxygen has a higher need than Carbon. Even though the carboxylic gathering may appear as though it is the most noteworthy need since it has more molecules, the hydroxyl bunch is a higher need for practical gathering. Supplant the COOH bunch with an NH2.
Q. What do you mean by atomicity?
A. Atomicity is characterised as the complete number of particles that establish an atom. For instance, every particle of oxygen (O2) is made out of two oxygen iotas.
Chemistry Alcohols, Phenols and Ethers Exam
Student Forum
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test