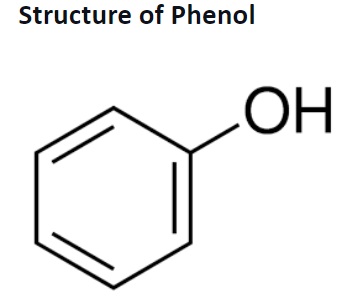
Phenols are crystalline white solid compounds that are volatile in nature. They were first extracted from coal tar and are formed when organic materials decompose.
What is a phenol?
Phenols or phenolics are chemical compounds that consist of a hydroxyl group (-OH) attached to an aromatic hydrocarbon. They are known as a subset of alcohol but exhibit different physical and chemical properties in comparison. They are also called carbolic acids and are used to prepare nylons, herbs, detergents, and other pharmaceutical products.
- FAQs regarding Phenol
FAQs regarding Phenol
Q. What are the different compounds used for the preparation of phenol?
Q. Who discovered phenol?
Q. Which is the simplest and the most commonly occurring phenol?
Q. How does phenol affect the human body?
Q. Where can phenols be found naturally?
Chemistry Alcohols, Phenols and Ethers Exam
Student Forum
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test