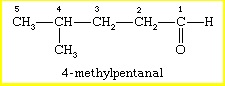Aldehyde Group
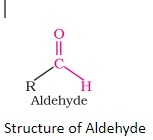
Aldehydes are organic compounds comprising a terminal carbonyl group. An aldehyde group is a functional group that has a carbon atom with -
- Single bond with a hydrogen atom
- Single bond with any other atom or groups of atoms
- Double bond with an oxygen atom
The double bond between carbon and oxygen is known as the carbonyl group and is an essential characteristic of aldehydes (>C=0). The aldehyde group is also referred to as the formyl or methanoyl group.
Aldehydes are derived from alcohols by the removal of hydrogen, also known as dehydrogenation. That is why the name aldehyde originates from ‘alcohol dehydrogenated.’
Structure of Aldehydes
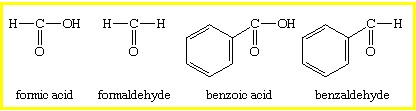
Formaldehyde is the simplest aldehyde where the carbonyl group bonds with two hydrogen atoms. In other aldehydes, the carbonyl group is paired with one hydrogen and one carbon group.
Properties of Aldehydes
Most of the aldehydes are liquid at room temperature. However, the exceptions are formaldehyde and methanal, which are gaseous at room temperature.
Boiling point: The boiling point of aldehydes is higher than that of hydrocarbons. Since aldehydes do not form H-bonds with themselves, the boiling point is comparatively lower than that of corresponding alcohols and carboxylic acids.
Solubility: Aldehydes such as methanol, ethanol, and propanone form a homogeneous mixture when mixed with water. This happens because the hydrogen atoms form a bond with water. However, the solubility decreases if the length of the alkyl chain increases. All aldehydes are soluble in organic solvents like ether, chloroform, benzene, etc.
Odour: The lower aldehydes have a pungent odour. However, the odour becomes more fragrant as the size of the molecule increases. Naturally occurring aldehydes are used as flavoring agents and even in the blending of perfumes.
Nomenclature of Aldehydes
There are two systems of naming aldehydes:
International Union of Pure and Applied Chemistry (IUPAC): According to this system, the longest chain of carbon atoms containing the carbonyl group is considered as the parent alkaline. The naming is done by changing the suffix -e to -al. For example, the compound 4-methylpentanal has five carbon atoms; hence the parent name is pentane. The suffix -al is used to denote that it’s an aldehyde group. Since the methyl group is bonded to the fourth carbon of the chain, it gets the number 4 in its name. IUPAC is also called systemic nomenclature.
Uses of Aldehydes
Aldehydes are used widely by chemists to synthesize other compounds. However, they are less commonly used to produce compounds on a large scale. Of all the aldehydes, only formaldehyde is produced on an industrial scale to make formalin. Formalin is used for preserving fruits and vegetables, tanning, and embalming. It is also used as an insecticide, germicide, and fungicide for plants.
Aldehydes are also used to make polymers, urea, and melamine. They are also used as flavouring agents, solvents, and perfumes.
Class 12 Aldehyde Group
You will learn about aldehydes in chapter 12 of your textbook. You will also learn about ketones and carboxylic acid in this chapter.
Illustrated examples
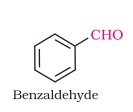
1. Draw the structure of Benzaldehyde.
Answer:
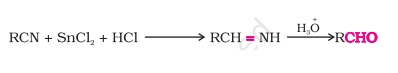
2. How is aldehyde prepared from nitriles and esters?
Answer: Nitriles are reduced to the corresponding imine with stannous chloride with hydrochloric acid, which on hydrolysis produces aldehyde.
FAQs on Aldehyde Group
Q: What is the functional group of aldehydes?
Q: What are the aldehydes that perform essential functions in humans and other living organisms?
Q: Which aldehydes naturally occur in flavouring agents?
Q: What is the common name of aldehydes?
Q: What is the difference between an aldehyde and a ketone?
News & Updates
Chemistry Aldehydes, Ketones and Carboxylic Acids Exam
Student Forum
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test