
Henry's Law is a fundamental principle in chemistry that describes the relationship between the concentration of a gas in a liquid and the partial pressure of that gas above the liquid's surface at a constant temperature. It is named after the British chemist William Henry, who formulated this law in the early 19th century. A quantitative relation between pressure and solubility of a gas in a solvent was first given by Henry, which is known as Henry’s law.
Henry's law states that at a constant temperature, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas present above the surface of liquid or solution. Henry’s law is an important topic in NCERT Class 12 Chemistry. Henry’s law is also stated as the partial pressure of the gas in vapour phase (p) is proportional to the mole fraction of the gas (x) in the solution.
We can express the law as: p = KH x
Here KH denotes the Henry’s law constant, p is the partial pressure of the gas in the vapour phase and x is the concentration of the dissolved gas. Students will learn this topic in chapter Solutions. In this article, students will learn Henry’s law statement, applications of Henry’s law, limitations etc.
- Factor Affecting Henry's Law Constant
- Henry’s Law: Applications
- Henry’s Law: Limitations
- FAQs on Henry's Law
Factor Affecting Henry's Law Constant
The factors that affect the Henry law constant are as follows.
- Nature of gas
- Nature of Solvent
- Pressure
- Temperature
Henry's Law: Important Points
- Direct Proportionality: Henry's Law states that the concentration of a gas dissolved in a liquid is directly proportional to the partial pressure of that gas above the liquid. In other words, if you increase the pressure of the gas above the liquid, more of the gas will dissolve into the liquid.
- Temperature Dependence: Henry's law constants are temperature-dependent. As temperature increases, the solubility of gases generally decreases, and vice versa. This means that the same gas will have different Henry's law constants at different temperatures.
- Ideal Behavior: Henry's Law assumes ideal behavior, meaning that there are no significant intermolecular forces between gas molecules or between gas molecules and the solvent molecules. In reality, deviations from ideal behavior can occur, especially at high pressures and low temperatures.
- Gas Concentration: Henry's Law allows us to calculate the concentration of a gas in a liquid by knowing the partial pressure of the gas and the Henry's law constant for that specific gas-solvent pair at a given temperature.
Henry’s Law: Applications
Following are the applications of Henry’s Law.
- The bottles are sealed under high pressure, to increase the solubility of CO2 in soft drinks and soda water.
- Deep-sea diving.
- It helps scuba divers to breathe underwater
- At high altitudes the partial pressure of oxygen is less than that at the ground level. This results in low concentrations of oxygen in the blood and tissues of people living at high altitudes or climbers. Low blood oxygen causes climbers to become weak and unable to think clearly, symptoms of a condition known as anoxia.
Henry's Law is relevant in various fields of science and engineering, including chemistry, biology, environmental science, and engineering.
Henry’s Law: Limitations
- Henry’s law does not hold true when gases are placed under extremely high pressure.
- This law is only applicable when the molecules of the system are in equilibrium state.
- The law is not applicable when the gas and the solution participate in chemical reactions with each other.
FAQs on Henry's Law
Q: Calculate the osmotic pressure in pascals exerted by a solution prepared by dissolving 1.0 g of polymer of molar mass 185,000 in 450 mL of water at 37°C.
A:
Given,
Volume of water, V = 450 mL = 0.45 L
Temperature, T=(37 + 273)K = 310 K
1.0 g of polymer of molar mass 185,000
Number of moles of polymer, n = 1 / 185,000 mol
We know that,
Osmotic pressure, π = nRT/V
= 1 X 8.314 X 103 X 310 / 185000 X 0.45
= 30.98 Pa
= 31 Pa (approx)
Q: Henry’s law constant for the molality of methane in benzene at 298 K is 4.27 × 10^5 mm Hg. Calculate the solubility of methane in benzene at 298 K under 760 mm Hg.
A:
Given-
Henry’s law constant KH = 4.27X 105 mm Hg,
p = 760mm Hg,
Using Henry’s law,

Using the formula of lowering vapour pressure,
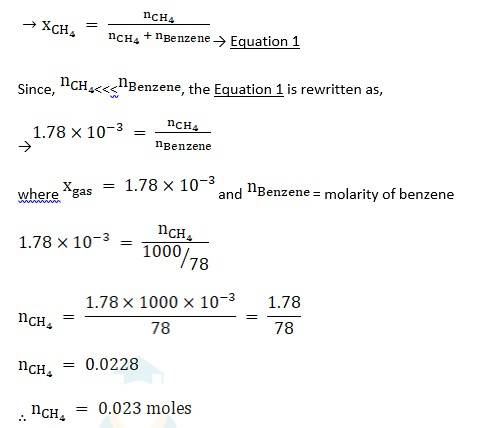
Thus, the solubility of methane in benzene is 0.023 moles
Q: he air is a mixture of a number of gases. The major components are oxygen and nitrogen with approximate proportion of 20% is to 79% by volume at 298 K. The water is in equilibrium with air at a pressure of 10 atm. At 298 K if the Henry’s law constants for oxygen and nitrogen at 298 K are 3.30 × 10^7 mm and 6.51 × 10^7 mm respectively, calculate the composition of these gases in water.
A:
Given-
KH for O2 = 3.30 × 107 mm Hg,
KH for N2 = 6.51 × 107 mm Hg
Percentage of oxygen (O2) = 20 %
Percentage of nitrogen (N2) = 79%
Total pressure = 10 atm
Using Henry’s law,

where, p is the partial pressure of gas in the solution and KH is Henry’s constant.

Thus, the mole fraction of oxygen in solution, xoxy = 4.61x10-5
and the mole fraction of nitrogen in solution, xnit is 9.22x10-5
Chemistry Atoms and Molecules Exam
Student Forum
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test
