In understanding the various physical and chemical properties of organic molecules, functional groups are very important. The carbon atom next to the double-bonded carbon atom is Allylic carbon. The carbon atom that is adjacent to the double bond can be identified as Allylic Carbon. This is the closest carbon atom to the double bond, but it is not a member of the double bond. In other words, this carbon atom is bound to a carbon atom that is doubly bonded to another carbon atom in exchange.
- Allylic Carbon Atoms
- Allylic Halides
- Allylic Carbonation
- Allyl Substitution of Alkenes
- FAQs on Allylic Carbons
Allylic Carbon Atoms
An allylic carbon atom is an sp3 hybridised carbon atom in the allylic group RCH2-CH=CH2 and is bonded to the -CH=CH2 group.
For e.g., the exposed carbon atom in propene is an allylic carbon atom (CH3-CH=CH2). Comparably, in cyclohexene, the carbon atoms adjacent to the double bond become allylic carbon atoms.
Allylic Halides
Compounds of halogen atoms bound to sp3-hybridized carbon to carbon-carbon double bond (C=C) i.e., to allylic carbon.
Allylic Carbonation
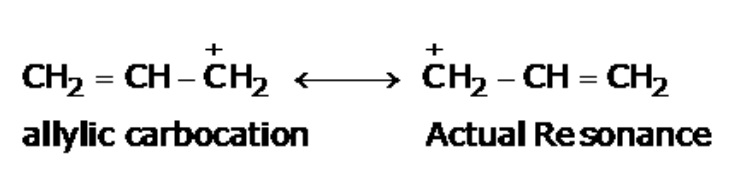
Allylic carbonation is actually a resonance-stabilized carbonium ion that has two resonance structures in which the formal charge of +1 is on an allylic carbon.
Allyl Substitution of Alkenes
An allylic rearrangement or an allylic transition is an organic reaction in which the dual bond of an allyl chemical compound moves to the next carbon atom. It is used for nucleophilic substitution.
The intermediate is a carbohydrate for which several resonance structures are possible under the reaction conditions that support the SN1 reaction method. This demonstrates the distribution (or spread) of the sample after recombination with nucleophilic Y. This step form is called the substitution of SN1.
Alternatively, nucleophiles may strike directly at the allylic position, displacing the leaving group in a single stage, in a process referred to as SN2 substitution. This is likely in situations when the allyl compound is unhindered and a good nucleophile is used. The goods would be identical to those used with the replacement of SN1. Thus, the interaction of 1-chloro-2-butene with sodium hydroxide results in a mixture of 2-buten-1-ol and 1-buten-3-ol.
Allylic Carbon in Class 10
Class 10 has a basic study of the allylic compound under the chapter Carbon and its Compound. Its weightage is 5 marks.
Allylic Carbon in Class 11
In the GOC part, you will learn the basics of organic chemistry and the stability order. The way allylic compounds and carbon react is also explained in Class 11. It has a weightage of 3.3% in JEE-mains.
Allylic Carbon in Class 12
Here you will get to apply the concepts of the allylic compound as in Class 12. You will have to study different compounds of organic chemistry. The first appearance happens in Chapter, Haloalkanes, and Haloarenes, and then it continues to be present in almost every chapter of organic. It has a weightage of 4 marks.
Illustrated Examples
- What is Vinyl and Allylic Carbon?
The distinction between allylic and vinyl carbon is that the carbon atom next to the double-bonded carbon atom is the allylic carbon, while the vinyl carbon atom is one of the two atoms that share the double bond.
- Why is vinyl carbonate unstable?
Owing to the disparity in the hybridization of the carbon-containing positive charge, the vinyl cations are less stable. They cannot donate more electrons to the positive charge found in the empty p-orbital because sp hybridized carbons have fewer p-characters, thereby destabilizing it.
- Which is more stable, allylic, or benzylic?
234 kcal/mol is benzyl cation, and 256 kcal/mol is allyl cation, so 22 kcal/mol is more "stable" benzyl cation.
FAQs on Allylic Carbons
Q. How can you tell if carbon is allylic?
Q. Why are allylic carbocations stable?
Q. Which Carbocation is most stabilized?
Q. What does allylic mean?
Q. What is allylic substitution?
Chemistry Carbon and its Compounds Exam
Student Forum
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test