NCERT Class 11 Solution Hydrocarbon: In Hydrocarbons Class 11 NCERT Solutions, students will learn about the structure and properties of all types of hydrocarbons. Students must learn NCERT Class 11 Chemistry Hydrocarbon Solutions to understand the chapter and score good marks in the exam. A lot of questions are asked from Class 11 Chemistry Hydrocarbons NCERT Solutions in exams like JEE Main and NEET as well as board exams. Students are thus advised to study Class 11 Chemistry Ch 13 NCERT Solutions thoroughly. Hydrocarbons Class 11 Exercise Solutions are provided here completely. The Chapter Hydrocarbons Class 11 NCERT Solutions provided here have been provided by subject experts and formulated in an easy-to-understand manner. Students preparing for entrance exams can refer to NCERT Solutions Chemistry class 12.
Hydrocarbons are chemical compounds consisting only of Hydrogen and carbon. Hydrocarbons are highly combustible and the main energy source presently available in abundance on Earth. Some examples of hydrocarbons are petroleum (crude oil), natural gas, coal, gasoline (petrol), methane, propane, paraffin wax, naphthalene and polymers. Hydrocarbons are primarily of three types; saturated hydrocarbons having a single bond (Alkane), unsaturated hydrocarbons and more than one bond (Alkene and Alkyne) and aromatic hydrocarbons (Cycloalkane).
- Topics Covered in Hydrocarbon Chapter
- Hydrocarbons Solutions and FAQs
Topics Covered in Hydrocarbon Chapter
Students can check below the list of all the topics that are covered in the Hydrocarbon chapter of NCERT class 11 Chemistry.
- Classification
- Alkanes
- Nomenclature and Isomerism
- Preparation
- Properties
- Conformations
- Alkenes
- Structure of Double Bond
- Nomenclature
- Isomerism
- Preparation
- Properties
- Alkynes
- Nomenclature and Isomerism
- Structure of Triple Bond
- Preparation
- Properties
- Aromatic Hydrocarbon
- Nomenclature and Isomerism
- Structure of Benzene
- Aromaticity
- Preparation of Benzene
- Properties
- Directive Influence of a Functional Group in Monosubstituted Benzene
- Carcinogenicity and Toxicity
HYDROCARBONS Additional Questions
Assertion and Reason:
Directions:
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
13.1. Assertion: Out of staggered and the eclipsed conformations of ethane, staggered conformation is more stable.
Reason: Hydrogen atoms are farthest apart in staggered conformation.
Answer: (a)
13.2. Assertion: There is very little difference of electronegativity between carbon and hydrogen atoms.
Reason: Due to the strong forces of interaction, the first four members, C1 to C4 are gases.
Answer: (c) Alkanes are almost non-polar molecules because of the covalent nature of C-C and C-H bonds and due to very little difference of electronegativity between carbon and hydrogen atoms. They possess weak van der Waals forces. Due to the weak forces, the first four members, C1 to C4 are gases, C5 to C17 are liquids and those containing 18 carbon atoms or more are solids at 298 K.
13.3. Assertion: Attack of electrophileresults in the formation of σ-complex or arenium ion.
Reason: Sigma complex or arenium ion has sp3hybridised carbon.
Answer: (b) Attack of electrophile results in the formation of σ-complex or arenium ion in which one of the carbon is sp3 hybridised. Sigma complex or arenium ion loses its aromatic character because delocalisation of electrons stops at sp3 hybridised carbon.
13.4. Assertion: Staggered conformation is less stable than the eclipsed conformation.
Reason: Rotation around C–C bond in ethane is not completely free.
Answer: (d) Of all the conformations of ethane, the staggered form has the least torsional strain and the eclipsed form, the maximum torsional strain. Therefore, staggered conformation is more stable than the eclipsed conformation. Hence, molecule largely remains in staggered conformation or we can say that it is preferred conformation. Thus, it may be inferred that rotation around C–C bond in ethane is not completely free.
13.5. Assertion: Alkenes are easily attacked by electrophilic reagents.
Reason: pi (π) bond makes alkenes behave as sources of loosely held mobile electrons.
Answer: (a) pi (π) bond is a weaker bond due to poor sideways overlapping between the two 2p orbitals. Thus, the presence of the pi (π) bond makes alkenes behave as sources of loosely held mobile electrons. Therefore, alkenes are easily attacked by reagents or compounds which are in search of electrons. Such reagents are called electrophilic reagents. The presence ofweaker π-bond makes alkenes unstable molecules in comparison to alkanes and thus, alkenes can be changed into single bond compounds by combining with the electrophilic reagents.
MCQs
13.1 preparation of higher alkanescontaining even number of carbonatoms is done via
(a)β-elimination reaction (b) Kharash effect (c) Wurtz reaction (d) Edwards reaction
Answer:(c) Wurtz reaction
13.2 Higher alkanes on heating to higher temperature decompose into lower alkanes,alkenes etc. Such a decomposition reaction into smaller fragments by the application of heat is called
(a) pyrolysis (b) cracking (c) both (d) none
Answer: (c) both
13.3 Which of the following statements is incorrect?
(a) Alkenes and alkynes undergo addition reactions, whichare mainly electrophilic additions.
(b) Alkanes show conformational isomerism due to free rotation along the C–C sigmabonds.
(c) Alkenes exhibit geometrical (cis-trans) isomerism due to restricted rotation around the carbon–carbon double bond.
(d) Aromatic hydrocarbons do not undergo electrophilic substitution reactions.
Answer: (d) Aromatic hydrocarbons, despite having unsaturation, undergo mainly electrophilic substitution reactions. These undergo addition reactions only under special conditions.
13.4. What happens when ethanol is heated with cone. H2SO4?
(a) Ethane is formed (b) methane is formed (c) propane is formed (d) ethene is formed
Answer: (d) ethene is formed
13.5. Baeyer’s reagent is
(a) aqueous KMnO4 (b) neutral KMnO4
(c) alkaline KMnO4 (d) aqueous bromine water
Answer: (c)
13.6. Which of the following is less reactive than benzene towards electrophilic substitution reactions?
(a) Nitrobenzene (b) Aniline
(c) Bromobenzene (d) Chlorobenzene
Ans: (a)
Questions and Answers
13.1. Give reasons for the following:
(i) alkanes are also called as paraffins.
(ii) benzene does not undergo addition reactions.
(iii) Alkenes are commonly known as olefins
Answer: (i) Paraffins means little affinity. Alkanes due to strong C—C and C—H bonds are relatively chemically inert.
(ii) Benzene does not undergo addition reactions due to delocalization of π -electrons which makes it highly stable.
(iii) Alkenes are commonly known as olefins because the lower members form oily products on treatment with chlorine or bromine.
13.2. Write the Physical properties of Aromatic hydrocarbons.
Answer: Aromatic hydrocarbons are non- polarmolecules and are usually colourless liquids or solids with a characteristic aroma. Aromatic hydrocarbons are immiscible with water but are readily miscible with organic solvents. They burn with sooty flame.
13.3. Explain Friedel-Crafts alkylation reaction.
Answer: When benzene is treated with an alkyl halide in the presence of anhydrous aluminium chloride, alkyl benzene is formed. This reaction is called as Friedel-Crafts alkylation reaction.
13.4. What is Huckel rule?
Answer: Benzene was considered as parent ‘aromatic’compound. Now, the name is applied to all the ring systems whether or not having benzene ring, possessing following characteristics:
(i) Planarity
(ii) Complete delocalisation of the π electrons in the ring
(iii) Presence of (4n + 2) π electrons in the ring, where n is an integer (n = 0, 1, 2, . . .).
This is referred to as Huckel rule.
13.5. What are carcinogenic hydrocarbons? How do they acquire this property?
Answer: Benzene and polynuclear hydrocarbons containing more than two benzene rings fused together are toxic and said to possess cancer producing (carcinogenic) property. Such poly-nuclear hydrocarbons are formed on incomplete combustion of organic materials like tobacco, coal and petroleum.They enter into human body and undergo various biochemical reactions and finally damage DNA and cause cancer.
13.6. Explain Cyclic polymerisation of ethyne for benzene formation.
Answer: Ethyne on passing through red hot iron tube at 873K undergoes cyclic polymerization. Three molecules polymerise to form benzene, which is the starting molecule for the preparation of derivatives of benzene, dyes, drugs and large number of other organic compounds. This is the best route for entering from aliphatic to aromatic.
13.7. Write about the physical properties of alkynes.
Answer: Physical properties of alkynes follow the same trend of alkenes and alkanes. First three members are gases, the next eight are liquids and the higher ones are solids. All alkynes are colourless. Ethyene has characteristic odour. Other members are odourless. Alkynes are weakly polar in nature. They are lighter than water and immiscible with water but soluble in organic solvents like ethers, carbon tetrachloride and benzene. Their melting point,boiling point and density increase with increase in molar mass.
13.8. What is ozonolysis of alkenes?
Answer: Ozonolysis of alkenes involves the addition of ozone molecule to alkene to form ozonide, and then cleavage of the ozonide by Zn-H2O to smaller molecules.This reaction is highly useful in detecting the position of the double bond in alkenes or other unsaturated compounds.
13.9. What does Markovnikov rule state.
Answer: The rule states that negative part of the addendum (adding molecule) gets attached to that carbon atom which possesses lesser number of hydrogen atoms.
13.10. Explain dehydrohalogenation of Alkyl halides.
Answer: Alkyl halides (R-X) on heating with alcoholic potash (potassium hydroxide dissolved in alcohol,say, ethanol) eliminate one molecule of halogen acid to form alkenes. This reaction is known as dehydrohalogenation i.e., removal of halogen acid. This is example of β-elimination reaction, since hydrogen atom is eliminated from the β carbon atom (carbon atom next to the carbon to which halogen is attached).
Hydrocarbons Solutions and FAQs
Q 13.1. How do you account for the formation of ethane during chlorination of methane?
A 13.1 Chlorination of methane is a free radical reaction which occurs by the following mechanism involving initiation, propagation and termination steps:
From the above mechanism, it is evident that during propagation step, CH3 free radicals are produced which may undergo three reactions, i.e., (i), (ii) and (iii). In the chain termination step, the two CH3 free radicals combine together to form ethane (CH3—CH3) molecule.
Q 13.2. Write IUPAC names of the following compounds:
A 13.2
(a) 2-Methykbuut-2-ene
(b) Pent-1-ene-3-yne
(c) But-1,3-diene
(d) 4-Phenylbut-1-ene
(e) 2-Methyl phenol
(f) 5-(2-Methylpropyl)decane
(g) 4-Ethyldeca-1,5,8-triene
Q 13.3 For the following compounds, write structural formulas and IUPAC names for all possible isomers having the number of double or triple bond as indicated: (a) C4H8 (one double bond) (b) C5H8 (one triple bond)
A 13.3
Q 13.4. Write IUPAC names of the products obtained by the ozonolysis of the following compounds: (i) Pent-2-ene (ii) 3, 4-Dimethylhept-3-ene (iii) 2-Ethylbut-l-ene (iv) 1-Phenylbut-l-ene.
A 13.4
Q 13.5. An alkene ‘A’ on ozonolysis gives a mixture of ethanal and pentan-3-one. Write structure and IUPAC name of ‘A’.
A 13.5 Step 1. Write the structure of the products side by side with their oxygen atoms pointing towards each other.
Step 2. Remove the oxygen atoms and join the two ends by a double bond, the structure of the alkene ‘A’ is
Q 13.6. An alkene ‘A’ contains three C – C, eight C – H σ bonds and one C – C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
A 13.6
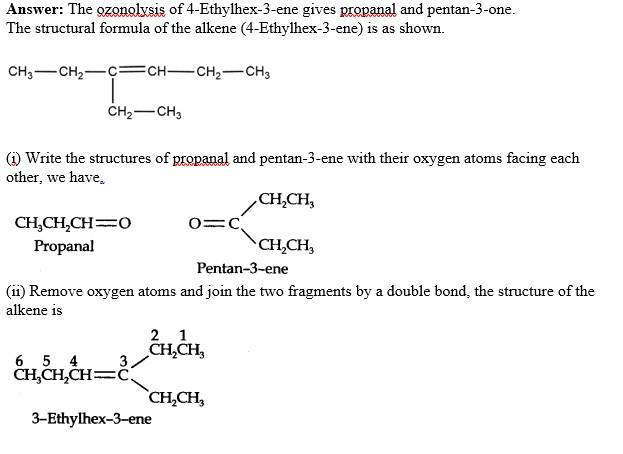
Q 13.7. Propanal and pentan-3-one are the ozonolysis products of an alkene. What is the structural formula of the alkene?
A 13.7
Q 13.8. Write chemical equations for the combustion reaction of the following hydrocarbons: (i) Butane (ii) Pentene (iii) Hexyne (iv) Toluene
A13.8 A combustion reaction is a reaction in which a substance reacts with oxygen gas, there is a formation of carbon dioxide, water with the evolution of light and heat.
Q 13.9. Draw the cis and trans structures for hex-2-ene. Which isomer will have higher b.p. and why?
A 13.9
Q 13.10. Why is benzene extraordinarily stable though it contains three double bonds?
A 13.10
Q 13.11. What are the necessary conditions for any system to be aromatic?
A 13.11
The necessary conditions for a molecule to be aromatic are:
- It should have a single cyclic cloud of delocalised n-electrons above and below the plane of the molecule.
- It should be planar. This is because complete delocalization of n-electrons is possible only if the ring is planar to allow cyclic overlap of p-orbitals.
- It should contain Huckel number of electrons, i.e., (4n + 2) n-electrons where n = 0, 1, 2, 3 etc.
A molecule which does not satisfy any one or more of the above conditions is said to be non-aromatic.
Q 13.12. Explain why the following systems are not aromatic?
A 13.12
Q 13.13. How will you convert benzene into (i)p-nitrobromobenzene (ii) m-nitrochlorobenzene (iii) p-nitrotoluene (iv) acetophenone?
A 13.13 (i) The two substituents in the benzene ring are present at p-positions. Therefore, the sequence of reactions should be such that first an o, p-directing group, i.e., Br atom should be introduced in the benzene ring and this should be followed by nitration. Thus,
Q 13.14. In the alkane, (given below) identify 1°, 2°, 3° carbon atoms and give the number of H atoms bonded to each one of these.
H3C—CH2—C(CH3)2—CH2—CH(CH3)2
A 13.14 The expanded formula of the given compound is
Q 13.15. What effect does branching of an alkane chain has on its boiling point?
A 13.15 Branching of carbon atom chain decreases the boiling point of alkane.
Q 13.16. Addition of HBr to propene yields 2-bromopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanism.
A 13.16 Addition of HBr to propene is an ionic electrophilic addition reaction in which the electrophile, i.e., H+ first adds to give a more stable 2° carbocation. In the 2nd step, the carbocation is rapidly attacked by the nucleophile Br~ ion to give 2-bromopropane.
Q 13.17 Write down the products of ozonolysis ofl, 2-dimethylbenzene (o-xylene). How does the result support Kekule structure of benzene?
A 13.17 o-Xylene may be regarded as a resonance hybrid of the following two Kekule structures. Ozonolysis of each one of these gives two products as shown below:
Thus, in all, three products are formed. Since all the three products cannot be obtained from any one of the two Kekule structures, this shows that o-xylene is a resonance hybrid of the two Kekule structures (I and II).
Q 13.18. Arrange benzene, n-hexane and ethyne in decreasing order of acidic behaviour. Also give reason for this behaviour.
A 13.18 The hybridization state of carbon in these three compounds is:
Since s-electrons are closer to the nucleus, therefore, as the s-character of the orbital making the C—H bond increases, the electrons of C—H bond lie closer and closer to the carbon atom. In other words, the partial +ve charge on the H-atom and hence the acidic character increases as the s-character of the orbital increases. Thus, the acidic character decreases in the order: Ethyne > Benzene > Hexane.
Q 13.19. Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
A 13.19 Benzene is a rich source of electrons because of the presence of an electron cloud containing 6 n-electrons above and below the plane of the ring. Consequently, it attracts the electrophiles (electron-deficient) reagents towards it and repels nucleophiles (electron- rich) reagents. As a result, benzene undergoes electrophilic substitution reactions easily and nucleophilic substitutions with difficulty.
Q 13.20. How would you convert the following compounds into benzene? (i) Ethyne (ii) Ethene (iii) Hexane.
A 13.20
Q 13.21. Write structures of all the alkenes which on hydrogeneration give 2-methylbutane.
A 13.21 The basic skeleton structure of 2-methylbutane is
Q 13.22. Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E+.
(a) Chlorobenzene, 2, 4-dinitrochlorobenzene, p-nitrochlorobenzene
(b) Toluene,p—H3C—C6H4—NO2, p—O2N—C6H4—NO2.
A 13.22
(a) The typical reactions of benzene are electrophilic substitution reactions. Higher the electron-density in the benzene ring, more reactive is the compound towards thesereactions. Since NO2 is a more powerful electron-withdrawing group than Cl, therefore, more the number of nitro groups, less reactive is the compound. Thus, the overall reactivity decreases in the order:
Chlorobenzene > p-nitrochlorobenzene > 2, 4-dinitrochlorobenzene
(b) Here, CH3 group is electron donating but NO2 group is electron-withdrawing. Therefore, the maximum electron-density will be in toluene, followed by p-nitrotoluene followed by p-dinitrobenzene. Thus, the overall reactivity decreases in the order:
Toluene >p—H3C—C6H4—NO2>p—O2N—C6H4—NO2.
Q 13.23. Out of benzene, m-dinitrobenzene and toluene which will undergo nitration most easily and why?
A 13.23 CH3 group is electron-donating while -NO2 group is electron-withdrawing. Therefore, maximum electron density will be in toluene, followed by benzene and least in m-dinitrobenzene. Therefore, the ease of nitration decreases in the order: toluene > benzene > m-dinitrobenzene.
Q 13.24. Suggest the name of a Lewis acid other than anhydrous aluminium chloride which can be used during ethylation of benzene.
A 13.24 Anhydrous Ferric Chloride (FeCl3) is another Lewis acid which can be used during ethylation of benzene.
Q 13.25. Why is Wurtz reaction not preferred for the preparation of alkanes containing odd number of carbon atoms? Illustrate your answer by taking one example.
A 13.25
Alkyl halides on treatment with sodium metal in dry ethereal (free from moisture) solution give higher alkanes. This reaction is known as Wurtz reaction and is used for the preparation of higher alkanes containing even number of carbon atoms.
For preparation of alkanes containing odd number of carbon atoms, a mixture of two alkyl halides has to be used. Since two alkyl halides can react in three different ways, therefore, a mixture of three alkanes instead of the desired alkane would be formed. For example, Wurtz reaction between 1-bromopropane and 1-bromobutane gives a mixture of three alkanes i.e., hexane, heptane and octane as shown below:
Explore exams which ask questions on Chemistry Ncert Solutions Class 11th
Select your preferred stream
Chemistry Ncert Solutions Class 11th Exam
Student Forum
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test