Kanishk FularaAssociate Senior Executive
NCERT Solutions for Class 11 Physics Chapter 12 Kinetic Theory are designed to help students prepare well for exams. The subject experts have provided in-depth explanations to help students understand complex concepts in NCERT Class 11 Physics Solutions. Learning from this page will help students to clarify their doubts and gain a better understanding of the subject.
The kinetic theory of gases postulates that gases exhibit certain behaviours based on the assumption that their composition comprises fast-moving atoms or molecules. The molecules in gases are loosely packed, with large intermolecular gaps between them. Through the kinetic theory of gases, the random movement of gas molecules can be explained.
Physics NCERT Class 11th Kinetic Theory: Topics Covered
- Introduction to Kinetic Theory
- Molecular nature of matter
- Behaviour of gases
- Kinetic theory of an ideal gas
- Law of equipartition of energy
- Specific heat capacity
- Mean free path
NCERT Physics Class11th Solution PDF for Kinetic Theory
NCERT Solution for Class XI Physics Chapter Kinetic Theory is available here in PDF format also. The candidates can click on the Physics NCERT Class 11 PDF link for the Kinetic Theory chapter given below and download it. The PDF format of solutions can be downloaded and used by the students to get their doubts cleared instantly.
Download Here: NCERT Solution for Class XI Physics Chapter Kinetic Theory PDF
Explore exams which ask questions on physics ncert solutions class 11th
Select your preferred stream
NCERT Physics Class11th Kinetic Theory Solutions and FAQs
Q.13.1 Estimate the fraction of molecular volume to the actual volume occupied by oxygen gas at STP. Take the diameter of an oxygen molecule to be 3 Å.
Ans.13.1 Diameter of the oxygen molecule, d = 3Å
Radius, r = = 1.5 Å = 1.5 cm
Actual volume occupied by 1 mole of oxygen gas at STP = 22.4 lit = 22400
Molecular volume of oxygen gas, V = N,
where N = Avogadro’s number = 6.023 molecules/mole
Therefore, V = 6.023 = 8.51
Ratio of the molecular volume to the actual volume of oxygen = = 3.8
Q.13.2 Molar volume is the volume occupied by 1 mol of any (ideal) gas at standard temperature and pressure (STP: 1 atmospheric pressure, 0 °C). Show that it is 22.4 litres.
Ans.13.2 The ideal gas equation relating to pressure (P), volume (V) and absolute temperature (T) is given by the relation
PV = nRT, where
R is the universal gas constant = 8.314 J /mol/K
n = number of moles = 1 (given)
T = standard temperature = 273 K
P = standard pressure = 1 atm = 1.013 N/
Therefore V = = = 0.02240 = 22.4 litres
Hence the molar volume of a gas at STP is 22.4 litres.
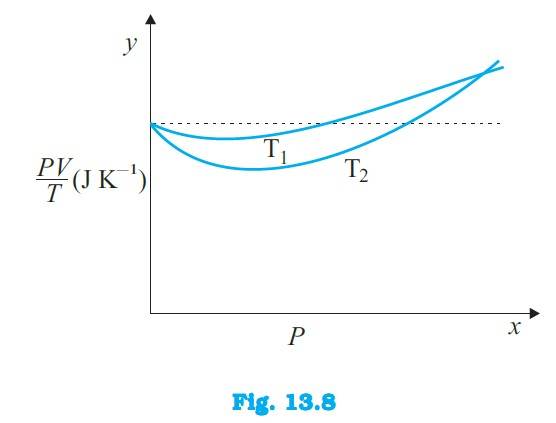
Q.13.3 Figure 13.8 shows plot of PV/T versus P for 1.00×10–3 kg of oxygen gas at two different temperatures.
(a) What does the dotted plot signify?
(b) Which is true: T1 > T2 or T1 < T2?
(c) What is the value of PV/T where the curves meet on the y-axis?
(d) If we obtained similar plots for 1.00×10–3 kg of hydrogen, would we get the same value of PV/T at the point where the curves meet on the y-axis? If not, what mass of hydrogen
yields the same value of PV/T (for low pressure high temperature region of the plot) ? (Molecular mass of H2 = 2.02 u, of O2 = 32.0 u, R = 8.31 J mo1–1 K–1.)
Ans.13.3 The dotted plot in the graph signifies the ideal behaviour of the
gas, i.e. = R, (where is the number of moles and R is the universal gas constant) is a constant quantity. It is not dependent on the pressure of the gas. The curve of the gas at temperature is closer to the dotted plot than the curve of the gas at temperature
A real gas approaches the behaviour of an ideal gas when its temperature increases. Therefore > is true for the given plot.
The value of the ratio , where the two curves meet, is R. This is because the ideal gas equation is given as = R. From the given data, we have
Molecular mass of oxygen = 32 g
Mass of oxygen = 1 = 1 g
R = 8.314 J/mole/K
Then = = 0.26 J/k
Hence, the value of the ratio , where the curves meet on the y-axis, is 0.26 J/K.
If we obtain similar plots for 1.00 kg of hydrogen, then we will not get the same values of at the point where the curves meet on the y-axis. This is because the molecular mass of hydrogen (2.02 u) is different from the oxygen (32.0 u). We have:
.
Given, molecular mass (M) of = 2.02 u
= R at constant temperature, where =
m = mass of
Then, m = = = 6.3 g = 6.3 kg
Hence, 6.3 kg of will yield the same value of
Q.13.4 An oxygen cylinder of volume 30 litres has an initial gauge pressure of 15 atm and a temperature of 27 °C. After some oxygen is withdrawn from the cylinder, the gauge pressure drops to 11 atm and its temperature drops to 17 °C. Estimate the mass of oxygen taken out of the cylinder (R = 8.31 J mol–1 K–1, molecular mass of O2 = 32 u).
Ans.13.4 Volume of oxygen, = 30 litres = 30
Gauge pressure, = 15 atm = 15 Pa
Temperature, = 27 = 300 K
Universal gas constant, R = 8.314 J/mole/K
Let the initial number of moles of oxygen gas cylinder be
From the gas equation, we get
= = 18.276
But = , where = initial mass of oxygen
M = Molecular mass of oxygen = 32 g
= M = 18.276 g
After some oxygen is withdrawn from the cylinder, the pressure and temperature reduces, but volume remained unchanged.
Hence, Volume, = 30 litres = 30
Gauge pressure, = 11 atm = 11 Pa
Temperature, = 17 = 290 K
Let the final number of moles of oxygen left in the cylinder be
From the gas equation, we get
= = 13.864
But = , where = remaining mass of oxygen
M = Molecular mass of oxygen = 32 g
= M = 13.864 g
So the mass of oxygen taken out = = 141.19 gm = 0.141 kg
Q.13.5 An air bubble of volume 1.0 cm3 rises from the bottom of a lake 40 m deep at a temperature of 12 °C. To what volume does it grow when it reaches the surface, which is at a temperature of 35 °C?
Ans.13.5 Volume of the air bubble = 1.0 = 1
Height achieved by bubble, d = 40 m
Temperature at a depth of 40 m, = 12 = 285 K
Temperature at the surface of the lake, = 35 = 308 K
The pressure at the surface of the lake, = 1 atm = 1.013 Pa
The pressure at 40m depth: = 1 atm + d
Where is the density of water = kg/
G = acceleration due to gravity = 9.8 m/
Hence = 1.013 = 493300 Pa
From the relation = , where is the volume of bubble when it reaches the surface, we get, = = 5.263
Q.13.6 Estimate the total number of air molecules (inclusive of oxygen, nitrogen, water vapour and other constituents) in a room of capacity 25.0 at a temperature of 27 °C and 1 atm pressure.
Ans.13.6 Volume of the room, V = 25.0
Temperature of the room, T = 27 = 300 K
Pressure of the room, P = 1 atm = 1 1.013 Pa
The ideal gas equation relating to pressure (P), volume (V) and absolute temperature (T) can be written as
PV = NT, where is Boltzmann constant = 1.38
N is the number of air molecules in the room.
N = = = 6.117
Q.13.7 Estimate the average thermal energy of a helium atom at (i) room temperature (27 °C), (ii) the temperature on the surface of the Sun (6000 K), (iii) the temperature of 10 million Kelvin (the typical core temperature in the case of a star).
Ans.13.7 At room temperature, T = 27 = 300 K
is Boltzmann constant = 1.38
Average thermal energy = = = 6.21 J
Hence, the average thermal energy of a helium atom at room temperature is 6.21 J
On the surface of the Sun, T = 6000 K
Hence average thermal energy = = = 1.242 J
Hence, the average thermal energy of a helium atom on the surface of the Sun is 1.242 J
Inside the core of a star, T = K
Hence average thermal energy = = = 2.07 J
Hence, the average thermal energy of a helium atom inside the core of a star is 2.07 J
Q.13.8 Three vessels of equal capacity have gases at the same temperature and pressure. The first vessel contains neon (monatomic), the second contains chlorine (diatomic), and the third contains uranium hexafluoride (polyatomic). Do the vessels contain equal number of respective molecules? Is the root mean square speed of molecules the same in the three cases? If not, in which case is vrms the largest?
Ans.13.8 According to Avogadro’s law, the three vessels will contain an equal number of the respective molecules. This number is equal to
Avogadro’s number, N = 6.023
The root mean square speed ( of a gas of mass m and temperature T is given by the relation . Where k is Boltzmann constant. For the given gases, k and T are constants. Hence depends only on the mass of the atoms Therefore, the root mean square speed of the molecules in the three cases is not the same. Among Neon, Chlorine and Uranium hexafluoride, the mass of the neon is the smallest, so Neon will have highest root mean square speed among the given gases.
Q.13.9 At what temperature is the root mean square speed of an atom in an argon gas cylinder equal to the rms speed of a helium gas atom at – 20 °C ? (atomic mass of Ar = 39.9 u, of He = 4.0 u).
Ans.13.9 Temperature of the helium atom, = – 20 °C = 253 K and temperature of argon atom be =
Atomic mass of helium, = 4.0 u
Atomic mass of Argon, = 39.9 u
Let be the rms speed of Argon and be the rms speed of Helium
From the relation of we get
rms speed of Argon,
rms speed of Helium,
Since both the speeds are equal, we get
= or = or = = = 2523.675 K = 2.523 K
Q.13.10 Estimate the mean free path and collision frequency of a nitrogen molecule in a cylinder containing nitrogen at 2.0 atm and temperature 17 . Take the radius of a nitrogen molecule to be roughly 1.0 Å. Compare the collision time with the time the molecule moves freely between two successive collisions (Molecular mass of = 28.0 u).
Ans.13.10 Pressure inside the cylinder containing nitrogen, P = 2.0 atm = 2 Pa
Temperature inside the cylinder, T = 17
Radius of nitrogen molecule, r = 1.0 Å = 1 m
Diameter of nitrogen molecule, d = 2 m
Molecular mass of nitrogen molecule, M = 28 u = 28 g (assume) = 28 kg
The root means square speed of nitrogen is given by the relation
R is the universal gas constant = 8.314 J/mole/K
Hence = 508.26 m/s
The mean free path is given by
where k = Boltzmann constant = 1.38 kg-
= 1.11 m
Collision frequency= = = 4.57 /s
Collision time, T = = s = 3.93 s
Time taken between successive collision T’ = = s = 2.18 s
= = 555.71
Hence, the time taken between successive collisions is 556 times the time taken for a collision.
Q.13.11 A metre long narrow bore held horizontally (and closed at one end) contains a 76 cm long mercury thread, which traps a 15 cm column of air. What happens if the tube is held vertically with the open end at the bottom?
Ans.13.11 Length of the narrow bore, L = 1 m = 100 cm
Length of the mercury thread, l = 76 cm
Length of the air column between mercury and the closed end, = 15 cm
Since the bore is held vertically in air with the open end at the bottom, the mercury length that occupies the air space is 100 –(76 + 15) = 9 cm
Hence, total length of the air column = 15 + 9 = 24 cm
Let h cm of mercury flow out as a result of atmospheric pressure.
Length of the air column in the bore = 24 + h cm
Length of the mercury column = 76 – h cm
Initial pressure, = 76 cm of mercury
Initial volume, = 15
Final pressure, = 76 – (76 – h) = h cm of mercury
Final volume, = (24 + h)
Since temperature remained constant throughout the process,
= or 76 = h
Or, + 24h - 76 =0
h = =
So h = 23.84 cm or -47.84 cm
Since height cannot be negative, so h = 23.84 cm
23.84 cm of mercury will flow out from the bore and (76-23.84) 52.16 cm of mercury will remain in the bore. The length of the air column will be 24 + 23.84 = 47.84 cm
Q.13.12 From a certain apparatus, the diffusion rate of hydrogen has an average value of 28.7 cm3 s–1. The diffusion of another gas under the same conditions is measured to have an average rate of 7.2 cm3 s–1. Identify the gas.
[Hint : Use Graham’s law of diffusion: R1/R2 = ( M2 /M1 )1/2, where R1, R2 are diffusion rates of gases 1 and 2, and M1 and M2 their respective molecular masses. The law is a simple consequence of kinetic theory.]
Ans.13.12 Rate of diffusion of hydrogen, = 28.7 cm3 s–1
Rate of diffusion of another gas, = 7.2 cm3 s–1
According to Graham’s law of diffusion, we have:
= , where = molecular mass of hydrogen = 2.02 g and is the molecular mass of the unknown gas
= 2.02 = 32.09 = Molecular mass of Oxygen
Hence, the unknown gas is Oxygen.
Q.13.13 A gas in equilibrium has uniform density and pressure throughout its volume. This is strictly true only if there are no external influences. A gas column under gravity, for example, does not have uniform density (and pressure). As you might expect, its density decreases with height. The precise dependence is given by the so-called law of atmospheres
n2 = n1 exp [ -mg (h2 – h1)/ kBT]
where n2, n1 refer to number density at heights h2 and h1 respectively. Use this relation to derive the equation for sedimentation equilibrium of a suspension in a liquid column:
n2 = n1 exp [ -mg NA ( - ) (h2 –h1)/ ( RT)]
where is the density of the suspended particle, and that of surrounding medium. [NA is Avogadro’s number, and R the universal gas constant.] [Hint : Use Archimedes principle to find the apparent weight of the suspended particle.]
Ans.13.13 According to law of atmospheres, we have
n2 = n1 exp [ -mg (h2 – h1)/ kBT] …..(i)
where is the number of density at height and is the number of density at height
mg is the weight of the particle suspended in the gas column
Density of the medium =
Density of the suspended particle =
Mass of one suspended particle = m’
Mass of medium displaced = m
Volume of the suspended particle = V
According to Archimedes’s principle for a particle suspended in a liquid column, the effective weight of the suspended particle is given as:
Weight of the medium displaced – weight of the suspended particle = mg – m’g
= mg- V = mg – ( )
= mg(1- ) ……(ii)
Gas constant R = N
…(iii)
Substituting from (ii) and (iii) in (i), we get
n2 = n1 exp [ -mg (h2 – h1)/ kBT]
= n1 exp
= n1 exp
Q.13.14 Given below are densities of some solids and liquids. Give rough estimates of the size of their atoms:
| Substance |
Atomic Mass (u) |
Density ( |
| Carbon ( Diamond) |
12.01 |
2.22 |
| Gold |
197.00 |
19.32 |
| Nitrogen (liquid) |
14.01 |
1.00 |
| Lithium |
6.94 |
0.53 |
| Fluorine (liquid) |
19.00 |
1.14 |
[Hint : Assume the atoms to be ‘tightly packed’ in a solid or liquid phase, and use the known value of Avogadro’s number. You should, however, not take the actual numbers you obtain for various atomic sizes too literally. Because of the crudeness of the tight packing approximation, the results only indicate that atomic sizes are in the range of a few Å].
Ans.13.14 Let the atomic mass of a substance be = M and the density of the substance be =
Avogadro’s number, N = 6.023
Volume of N number of molecules = ……..(i)
Volume of one mole of a substance = ……(ii)
Equating (i) and (ii), we get
r =
For diamond, M = 12.01 kg, = 2.22 kg/
= = 1.29 Å
For gold, M = 197 kg, = 19.32 kg/
= = 1.59 Å
For liquid nitrogen, M = 14.01 kg, = 1.0 kg/
= = 1.77 Å
For lithium, M = 6.94 kg, = 0.53 kg/
= = 1.73 Å
For fluorine, M = 19 kg, = 1.14 kg/
= = 1.88 Å
News & Updates
physics ncert solutions class 11th Exam
Student Forum
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test