NCERT Class 12 Chemistry Alcohol, Phenol and Ethers Solution: The Alcohol, Phenol and Ethers chapter is an important chapter for class 12 board exams as well as competitive exams like JEE. The candidates can check the NCERT Class 12 Chemistry Solutions for Alcohol, Phenol and Ethers here.
Alcohols and phenols are formed when a hydrogen atom in a hydrocarbon, aliphatic and aromatic respectively, is replaced by –OH group. The substitution of a hydrogen atom in a hydrocarbon by an alkoxy or aryloxy group (R–O/Ar–O) yields another class of compounds known as ‘ethers’, for example, CH3OCH3 (dimethyl ether).
These classes of compounds find wide applications in industry as well as in day-to-day life. For instance, the ordinary spirit used for polishing wooden furniture is chiefly a compound containing hydroxyl group, ethanol. The sugar we eat, the cotton used for fabrics, the paper we use for writing, are all made up of compounds containing –OH groups.
Topics Covered in NCERT Chemistry Class 12 Alcohol, Phenol and Ethers Chapter
Candidates can check here all the topics covered in Alcohol, Phenol and Ethers chapter of NCERT class 12 Chemistry.
- Classification
- Alcohols and Phenols (Mono, Di, Tri or Polyhydric Compounds)
- Ethers
- Nomenclature
- Structures of Functional Groups
- Alcohols and phenols
- Preparation of Alcohols
- Preparation of Phenols
- Physical Properties
- Chemical Reactions
- Some Commercially Important alcohols
- Ethers
- Preparation of Ethers
- Physical Properties
- Chemical Reactions
NCERT Chemistry Class 12th Solution PDF - Alcohol, Phenol and Ether Chapter Download
The Alcohol, Phenol and Ether chapter is an important chapter for class 12 board exams as well as competitive exams like JEE Mains. Candidates are provided here NCERT Chemistry class 12 Alocohol, Phenol and Ether solutions.
The students can download here the Chemistry NCERT Class 12 PDF with solutions for free.
Download Here: NCERT Solution for Class XII Chemistry Alcohol, Phenol and Ether PDF
Alcohol, Phenol and Ethers Solutions and FAQs
Get here all the questions of NCERT Class 12th Alcohol Phenol and Ethers Chapter.
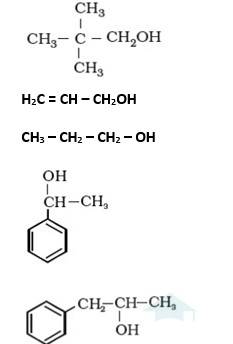
Q 11.1 Classify the following as primary, secondary and tertiary alcohols:
- A11.1 It is primary alcohol because carbon which carries the –OH group is only attached to one alkyl group.
- It is primary alcohol because carbon which carries the –OH group is only attached to one alkene group.
- It is primary alcohol because the carbon which carries the –OH group is only attached to one propyl group.
- It is secondary alcohol because the carbon which carries the –OH group is joined directly to methyl and benzene.
- It is secondary alcohol because the carbon which carries the –OH group is joined directly to two different alkyl groups.
Q 11.2 Identify allylic alcohols in the above examples.
A 11.2
Allylic alcohol is an organic compound which has the structural formula CH2 = CHCH2OH. In other words, in these alcohols, the the-OH group is attached to sp2 hybridized carbon next to the carbon-carbon double bond, that is to an allylic carbon. Therefore, in the above examples, the following are the allylic alcohols.
(ii) H2C = CH – CH2OH and
Q 11.3 Name the following compounds according to IUPAC system.
A 11.3
- 3-chloroethyl-2-isopropylpentan-1-ol
- 2,5-Dimethylhexane-1,3-diol
- 3-Bromocyclohexanol
- Hex-1-en-3-ol
- 2-Bromo-3-methylbut-2-en-1-ol
Q 11.4 Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?
A 11.4
In the Grignard reagent reaction, the first step of the reaction is the nucleophilic addition of Grignard reagent to the carbonyl group to form an adduct, Hydrolysis of adduct results in the formation of alcohol.
Here, is the general reaction with Grignard reagent below:-
From here, it is clear that HCHO gives CH2OH groups, so R of Grignard reagent is the remaining part of given alcohols. Thus, select the suitable Grignard reagent by substituting the value of R. Now we can see the reaction given below:-
Methanal reacts with iso-propyl magnesium bromide, in presence of dry ether gives an additional compound. And this additional compound on reaction with H2O /H+ gives iso-butyl alcohol (i.e., 2-methylpropane-1-ol) as one of the final product.
Methanal reacts with cyclohexyl magnesium bromide, in presence of dry ether, which gives an intermediate product. This intermediate product when reacts with given reagent, as shown above, gives cyclohexyl methanol as a product.
Q 11.5 Write structures of the products of the following reactions:
- A 11.5 In this reaction, when propene reacts with the given reagent then the double bond of propene breaks down with charges on them. So, H+ gets placed on the carbon which already has two hydrogen atom and OH- gets substituted on center carbon because it has the more positive charge which attracts OH-. Thus we get propene-2-or as a
- In this reaction, when Methyl ( 2-oxocyclohexyl) ethanoate reacts with the given reagent then the double bond between the oxygen atom and cyclohexyl gets breaks down, such that O has a negative charge and that particular carbon will have a positive charge on it. So, to neutralize it, H+ gets substituted to that carbon and with O- to form the structure of alcohol. Thus we get Methyl (2-hydroxycyclohexyl) ethanoate as a
- In this reaction, when 2-Methylbutanal reacts with the given reagent NaBH4, then the double bond between carbon and oxygen gets to break down in the above aldehyde compound, where carbon has positive charge and oxygen will have a negative charge on So, H+ gets placed on the carbon to complete its octet and H+ gets substituted on O- to form OH, i.e, an alcohol. Thus we get 2-Methylbutan-1-ol as a product.
Q 11.6 Give structures of the products you would expect when each of the following Methylbutan alcohol reacts with (a) HCl –ZnCl2 (b) HBr and (c) SOCl2.
A 11.6
(a)(i) Primary alcohols do no react appreciably with Lucas’ reagent (HCl –ZnCl2) at room temperature.
(ii) Tertiary alcohol reacts immediately with Lucas ‘reagent.
Q 11.7 Predict the major product of acid catalysed dehydration of (i) 1-methylcyclohexanol and (ii) butan-1-ol
A 11.7
Q 11.8 Ortho and para nitrophenols are more acidic than phenol. Draw the resonance structures of the corresponding phenoxide ions.
A 11.8
Resonating structures of o-nitrophenoxide ions that are formed by the loss of a proton from o-nitrophenol are as follows:
Resonating structures of p-nitrophenoxide ions that are formed by the loss of a proton from p- nitrophenol are as follows:
Resonating structures of phenoxide ions that are formed by the loss of a proton from phenol are as follows:
It is clearly evident from the above structures that due to —R-effect of— NO2NO2 group, o-and p-nitrophenoxide ions are more stable than phenoxide ions. Consequently, o- and p- nitrophenols are more acidic than phenols.
Q 11.9 Write the equations involved in the following reactions: (i) Reimer - Tiemann reaction (ii) Kolbe’s reaction
A 11.9
- Phenol on mixing with chloroform and NaOH at 340K followed by Acidic hydrolysis, salicyl aldehyde is formed. When carbon tetrachloride (CCl4) is used at the place of chloroform salicylic acid is This type of reaction is known as Reimer - Tiemann reaction.
- The sodium phenoxide reacts with CO2 under pressure 4-7 atm at a 400K temperature to form sodium salicylate, which on acidification yields salicylic acid. This type of reaction is known as Kolbe’s
Q 11.10 Write the reactions of Williamson synthesis of 2-ethoxy-3-methylpentane starting from ethanol and 3-methylpentan-2-ol.
A 11.10
During Williamson synthesis of ethers, an alkyl halide reacts with an alkoxide(ion with –ve charge on the oxygen of alcohol and + ve charge on alkali metal like Na) ion. it is an SN2 reaction. In the reaction, alkyl halides should be least hindered. Hence, an alkyl halide is obtained from ethanol and alkoxide ion from 3-methylpentan-2-ol. The reactions are shown below:
Q 11.11 Which of the following is an appropriate set of reactants for the preparation of 1- methoxy-4-nitrobenzene and why?
A 11.11 Set (ii) is appropriate Because CH3Br is only a nucleophile whereas CH3ONa is nucleophile as well as strong base, so the elimination reaction can occur,
Q 11.12 Predict the products of the following reactions:
A 11.12
- When N –propyl methyl ether reacts with HBr, it forms propanol and bromomethane, n- propyl methyl ether will cleave at O . and H+ will attack at O, and Br- will attack CH +
- When Ethoxybenzene Reacts with HBr, it forms Phenol and bromoethane, Ethoxybenzene cleaves at H+ will attack at O, and Br- will attack C H +
- When nitrating mixture reacts with ethoxy benzene introduction of nitro group is occurred at para position as it will give the stable product without
- As HI is a strong nucleophile it will protonate the oxygen , to form a good leaving And I- will attack at C(CH3)3+ to give tert- butyl iodide and ethanol.
Exercise Q 11.1 Write IUPAC names of the following compounds:
A 11.1
1. 2,2,4-Trimethylpentan-3-ol
The naming of the compound usually starts with numbering the carbons in the chain. The lower set of locants are chosen for this purpose, while in this case numbering under this condition is done from the left side. Once the carbons are mentioned, the position of -OH group is numbered and -ol is added as the suffix.
2. 5-Ethylheptane-2,4-diol
Here the longest chain is the straight chain. For such situations, numbering should be such that the functional groups should be denoted by the smallest number. Ethyl group is named at the beginning as it’s a side chain.
3. Butane-2,3-diol
In this system, glycols are called as Diols and their class name is Alkane diols. The two-hydroxyl group position is indicated by Arabic numerals. Firstly, the carbon chain is named, the -OH positions are numbered and suffixed with -diol (depends on the number of -OH groups.
4. Propane-1,2,3-triol
Initially carbon chain is named, -OH groups are numbered (can be done from any side as it’s a symmetrical compound) and suffixed with triol.
5. 2-Methylphenol
In the IUPAC system, the position of the substituent w.r.t –OH group is indicated by an Arabic numeral, with the carbon carrying the OH group being numbered 1. So, methyl is numbered at the minimum position and phenol is added. The common name is o-cresol (ortho-cresol).
6. 4-Methylphenol
Methyl group is numbered 4 w.r.t to -OH position. The compound is symmetrical. The compound is known by its common name p-cresol (para cresol).
7. 2,5-Dimethylphenol
The position of the substituent w.r.t –OH group is indicated by an Arabic numeral, with the carbon carrying the OH group being numbered 1. The lowest number chain is further chosen. The positions of the methyl group is 2 and 5.
8. 1-Methoxy-2-methylpropane
Longest carbon chain attached to oxygen is chosen. It is called the principal chain and the other carbon chain is named with -oxy(Alkoxy) suffix at the end. Now the position of alkoxy group is 1
w.r.t the principal chain(propane) and the methyl group
Is 2. Further alphabetically methoxy comes before methyl, therefore named as such.
9. Ethoxybenzene
The principal chain is benzene, so the short chain is named as ethoxy and suffixed with benzene
10. 1-Phenoxyheptane
Principal chain is heptane group having 7 carbon groups. Phenol group is called as substituent alkoxy group and is named.
11. 2-Ethoxybutane
The longest chain here is butane and the ethyl group is attached to the 2nd carbon.
Q 11.2 Write structures of the compounds whose IUPAC names are as follows: (i) 2- Methylbutan-2-ol (ii) 1-Phenylpropan-2-ol (iii) 3,5-Dimethylhexane –1, 3, 5-triol (iv) 2,3 – Diethylphenol (v) 1 – Ethoxypropane (vi) 2-Ethoxy-3-methylpentane (vii) Cyclohexylmethanol (viii) 3-Cyclohexylpentan-3-ol (ix) Cyclopent-3-en-1-ol (x) 4-Chloro-3-ethylbutan-1-ol.
A 11.2 Butane is the longest chain.
Propane is principle chain and substituents are numbered accordingly.
Hexane is the longest chain. There are three -OH substituents and 2 methyl groups.
-OH of phenol is numbered a 1.
Propane is the principle chain and the alkoxy group is ethyl group.
Longest chain is pentane and substituents are numbered accordingly.
In such cases, cyclo groups are named first, followed by conventional naming methods.
The cyclo group is named first, followed by conventional naming methods.
Cyclo group is named first. Pentane having a double bond is named then with the suffix -OL added to the end.
The longest chain is butane when looked for minimum numbering case.
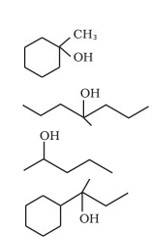
Q 11.3 (i) Draw the structures of all isomeric alcohols of molecular formula C5H12O and give their IUPAC names. (ii) Classify the isomers of alcohols in question 11.3 (i) as primary, secondary and tertiary alcohols.
A 11.3 The structures of all isomeric alcohols of C5H12O are given below:
Naming is done by the conventional method. The -OH group is attached on the first carbon.
(b) 3-Methylbutan-1-ol
Butane is the longest chain and methyl is the substituent group.
(c) 3-Methylbutan-1-ol
Longest chain is butane and conventional naming method is used.
(d) 2,2-Dimethylpropan-1-ol
Isomer is made by transforming the principal carbon into tertiary type. The longest chain is butane and named accordingly.
(e) Pentan-2-ol
Longest chain is pentane and the numbering is chosen from the minimum position.
(f) 3-Methylbutan-2-ol
Butane is the longest chain, further numbering is done by choosing a minimum position for the
-OH group followed by other substituents.
(g) Pentan-3-ol
Conventional method of naming is used.
(h) 2-Methylbutan-2-ol
Butane is the longest chain and substituents are named accordingly by looking into minimum position.
|
(A) Primary alcohol |
Explanation |
|
(i) Pentan-1-ol
(ii) 2-Methylbutan-1-ol
(iii) 3-Methylbutan-1-ol
(iv) 2,2-Dimethylpropan-1-ol |
The -OH group is attached to the carbon that is attached to another single carbon. |
|
(B) Secondary alcohol |
|
|
(i) Pentan-2-ol
(ii) 3-Methylbutan-2-ol
(iii) Pentan-3-ol |
The -OH group is attached to the carbon that is attached to another two carbons. |
|
(C) Secondary alcohol |
|
|
(i) 2-Methylbutan-2-ol |
The -OH group is attached to the carbon group that is further attached to another three carbon atoms. |
Q 11.4 Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
A 11.4
- Here, propanol undergoes intermolecular H-bonding because of the presence of -OH group while butane has no such property
(intermolecular Hydrogen bonding in propanol)
Therefore, extra energy will be required to break those hydrogen bonds which in turn causes higher boiling point for propanol when compared to butane.
Q 11.5 Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
A 11.5 Due to the presence of –OH group, alcohols form hydrogen-bonds with water but hydrocarbons cannot form hydrogen-bonds with water.
Due to inter moleculer hydrogen bonding between Alcohol and water molecular they remain tightly bounded to water molecules and have higher solubility. Whereas in case of hydrocarbon there is no chance of hydrogen bonding.
Q 11.6 What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
A 11.6
The hydroboration-oxidation reaction is a two-step reaction that converts an alkene into a neutral alcohol by the net addition of water across the double bond. The hydrogen and hydroxyl group are added in a syn addition leading to the cis configuration. Hydroboration- oxidation is an anti-Markovnikov reaction, with the hydroxyl group attaching to the less substituted carbon. In first step Addition of Hydroborate group is done and in next step, it is oxidized by hydrogen peroxide.
For example: - When propene undergoes hydroboration-oxidation reaction, then it produces propan-1-ol as product. In this reaction diborane i.e., (BH3)2 reacts with propene, which in result generates trialkyl borane as an addition product. Then trialkyl borane is oxidised, by using hydrogen peroxide in the presence of aqueous sodium hydroxide to form alcohol, as final product.
11.7 Give the structures and IUPAC names of monohydric phenols of molecular formula, C7H8O.
A 11.7 The different forms of cresol is formed with given molecular formula:
- 2-methylphenol
- 3-methylphenol
- 4-methylphenol
Q 11.8 While separating a mixture of ortho and para nitrophenols by steam distillation, name the isomer which will be steam volatile. Give reason
A 11.8 In ortho nitrophenol there is intra-molecular H bonding, whereas in para-nitrophenol there is inter-molecular H bonding, as shown below:
And because of that para-nitrophenol get tightly bounded with water and ortho nitrophenol is steam volatile and it will leave the solution.
Q 11.9 Give the equations of reactions for the preparation of phenol from cumene.
A 11.9 The conversion of Phenol from Cumene requires the air oxidation of
The air oxidation of cumene (isopropyl benzene) leads to the production of both phenol and acetone (costlier than phenol).
The air oxidation of cumene gives cumene hydro peroxide as an intermediate which on further hydrolysis (H3O+) gives phenol and acetone.
Q 11.10 Write chemical reaction for the preparation of phenol from chlorobenzene.
A 11.10 There are many ways to this conversion. Two of them are given below:-
(a) In the above conversion, the chlorobenzene is treated with a base such as NaOH, KOH etc. (strong base). The base abstracts the hydrogen from the C-2 position (it can also abstract the hydrogen from the C-6 position, as both are equally acidic) leaving the negative charge at that position.
In the next step Cl- leaves, leaving behind the positive charge at that carbon. Both the negative charge and positive charge forms a bond resulting Benzyne as the intermediate.
After the formation of the Benzyne intermediate OH- of the base attacks at the C-1 position and further, the H+ attacks to stabilize the negative charge thus resulting in the phenol.
The reaction can start from benzene also. Chlorination of benzene gives Chlorobenzene. When it is further treated with NaOH and H2O at 350°C it results in the formation of sodium phenoxide which on further treatment H+ gives Phenol as the final product.
Q 11.11 Write the mechanism of hydration of ethene to yield ethanol
A 11.11 Step 1:- Protonation of ethene to form carbocation by electrophilic attack of H3O+.
Step 2:-Nucleophilic attack of water on carbocation.
Step 3:- Deprotonation to form ethanol.
Q 11.12 You are given benzene, conc. H2SO4 and NaOH. Write the equations for the preparation of phenol using these reagents.
A 11.12 The reaction given below is:
Benzene reacts with concern. H2SO4 and undergoes the following mechanism:-
Step 1: The equilibrium produces SO3 in concentrated H2SO4, as shown below:
Step 2: SO3 is the electrophile which reacts with benzene to form arenium ion, as shown below:
Step 3: A proton is removed from the arenium ion to form benzenesulfonate ion.
Step 4: The benzenesulfonate ion accepts a proton to become benzene-sulphonic acid, as shown below:
Step 5: The benzene sulphonic acid then reacts with NaOH to give phenol as the final product, as shown below:
Q 11.13 Show how will you synthesise: (i) 1-phenylethanol from a suitable alkene. (ii) cyclohexylmethanol using an alkyl halide by an SN2 reaction. (iii) pentan-1-ol using a suitable alkyl halide?
A 11.13
- 1-phenylethanol from a suitable - The addition of water takes place according to Markovnikov rule. The alkene taken is styrene. And According to the rule, the positive charge i.e. H+ goes to the carbon of the double bond which has more number of hydrogens and the negative part i.e. OH- goes to the carbon that has less number of hydrogens. Therefore resulting the final product as 1-phenyl ethanol.
2.In the above conversion, NaOH gets dissociated into Na+ and OH-and Na+ then combines with Cl of chloromethylcyclohexane forming NaCl and thus the final product Cyclohexylmethanol is obtained.
3.In this conversion also, Na forms the compound with Cl i.e. NaCl and to compensate the negative charge formed by Cl, OH attacks at that position resulting in pentan-1-ol.
Q 11.14 Give two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of ethanol.
A 11.14 The acidic nature of phenol can be represented by the following two reactions:-
(a) Phenols react with sodium to give sodium phenoxide, liberating H2.
(b) Phenols react with sodium hydroxide to give sodium phenoxide and water as a by-product.
The acidity of phenol is more than that of ethanol. This is because phenol after losing a proton becomes phenoxide ion which undergoes resonance and is stabilized whereas ethoxide ion does not.
The resonating structures of phenoxide ion are shown as below:
The lone pair of electrons on oxygen delocalizes into the benzene (mesomeric effect) which reduces the electron density in the O-H bond. The O-H bonds are weaker and therefore breaks easily whereas in ethanol the electron releasing inductive effect of the alkyl group increases the electron density on the O-H bond. This strengthens the bond so the bond does not break easily. Therefore making ethanol less acidic than phenol.
Q11.15 Explain why is ortho nitrophenol more acidic than ortho methoxyphenol ?
A 11.15 ortho-nitro phenol is more acidic than ortho-methoxy phenol.
Explanation: Due to strong –R and –I effect of NO2 group, electron density in the O-H bond decreases and hence the loss of a proton becomes easy.
Now after the loss of a proton, the o-nitrophenoxide ion left behind is stabilized by resonance and thus making o-nitro phenol a stronger acid.
In contrast, due to the +R effect of methoxy group increases the electron density in the O-H bond. Thereby making the loss of proton difficult.
Now, the o-methoxyphenoxide ion left after the loss of a proton is destabilized by resonance. The two negative charges repel each other, thereby destabilizing the o-methoxyphenoxide ion.
Therefore, o-nitrophenol is more acidic than o-methoxyphenyl.
Q 11.16 Explain how does the –OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
A 11.16 The –OH group is an electron donating group. Thus, it increases the electron density in the benzene ring as shown by its resonating structure of phenol.
As a result benzene ring is activated towards electrophilic substitution.
Q 11.17 Give equations of the following reactions: (i) Oxidation of propan-1-ol with alkaline KMnO4 solution. (ii) Bromine in CS2 with phenol. (iii) Dilute HNO3 with phenol. (iv) Treating phenol with chloroform in presence of aqueous NaOH.
A 11.17 Oxidation of propane-1-ol with alkaline KMnO4 solution gives propanoic acid as the product. As the oxidation of primary alcohol gives carboxylic acid as the major product in the presence of a strong oxidizing reagent. And here KMnO4 is a very strong oxidizing agent.
A mixture of o-bromo phenol and p-bromo phenol is formed.
The formation of 2 products depends totally on the reaction conditions.
Dilute HNO3 with phenol.
Only dilute acid will be required for the nitration of phenol, nitric acid contains a small amount of nitrous acid which because of the activation of the ring will be more than enough to nitrate the phenol. Two products are formed 2-nitrophenol (ortho) and 4-nitrophenol (para).
Treating Phenol with chloroform in presence of aqueous NaOH results in the formation of salicylaldehyde(Reimer-Tiemann reaction)
Q 11.18 Explain the following with an example. (i) Kolbe’s reaction. (ii) Reimer-Tiemann reaction. (iii) Williamson ether synthesis. (iv) Unsymmetrical ether.
A 11.18 Kolbe’s Reaction: it is a carboxylation chemical reaction that proceeds by heating sodium phenoxide(the sodium salt of phenol)with carbon dioxide under pressure(100 atm,125°C), then treating the product with a sulphuric acid. The final product is salicylic acid (the precursor to aspirin).
The reaction is given as:
The mechanism is given below:
Reimer-Tiemann reaction: The Reimer Tiemann reaction is a chemical reaction used for the ortho-formylation of phenols, with the simplest example being the conversion
of phenol to salicylaldehyde.
When phenol is treated at 340K with chloroform and alkali, it forms salicylaldehyde.
Williamson ether synthesis: It is an organic reaction forming ether from an organohalide and a deprotonated alcohol (alkoxide). Typically it involves the reaction of an alkoxide ion with primary alkyl halide via SN2 reaction.
During this reaction, the main bonds broken is the C-Br bond and the new bonds formed are “C- O” bond.
Unsymmetrical ether: It is an ether in the molecule of which the two ligands on the ether group are different.
Eg.
Q 11.19 Write the mechanism of acid dehydration of ethanol to yield ethene.
A 11.19 The mechanism of acid dehydration of ethanol to yield ethane involves three steps:
Step 1:- Protonation of ethanol to form ethyl oxonium ion.
Step 2:- Formation of carbocation (rate determining step).
Step 3:-Elimination of a proton to form ethane
The acid consumed in step 1 is released in step 3. After the formation of ethane, it is removed to shift the equilibrium in a forward direction.
Q 11.20 How are the following conversions carried out? (i) Propene → Propan-2-ol. (ii) Benzyl chloride → Benzyl alcohol. (iii) Ethyl magnesium chloride → Propan-1-ol. (iv) Methyl magnesium bromide → 2-Methylpropan-2-ol.
A 11.20
- The conversion of Propene to propane-2-ol takes place according to markovnikoff rule. The positive part of H2O that is H+ goes to the carbon which has more hydrogen and the negative part that is OH-goes to carbon that has less number of carbons
- NaOH act as a base in the conversion of benzyl chloride to benzyl On hydrolysis removal of NaCl takes place and OH is inserted in place of Cl.
- Ethyl magnesium chloride (Grignard reagent) attacks on the carbon of the (The partial positive and negative charge is because of the electronegativity difference). After the formation of the addition product, hydrolysis takes place which further results in the formation of propane-1-ol and Mg(OH)Cl as the by product.
- Attack of methyl magnesium bromide (Grignard reagent) on carbonyl carbon results in the formation of adduct, which has partial charges due to electronegativity differences. The adduct on hydrolysis yields 2-methylpropan2-ol.
Q 11.21 Name the reagents used in the following reactions: (i) Oxidation of a primary alcohol to carboxylic acid. (ii) Oxidation of a primary alcohol to aldehyde. (iii) Bromination of phenol to 2,4,6-tribromophenol. (iv) Benzyl alcohol to benzoic acid. (v) Dehydration of propan-2-ol to propene. (vi) Butan-2-one to butan-2-ol.
A 11.21
- Acidified KMnO4 (potassium permanganate)
Potassium permanganate is a strong oxidant and is able to react with many functional groups. Here KMnO4 will readily react with primary carbon (where hydrogen is attached) and transforms that to acid.
- PCC (Pyridinium Chlorochromate)
This is actually a milder version of chromic acid. This works as a sort of elimination reaction. The formation of aldehyde occurs because of the action chromium (a good leaving group) which will be replaced when the C-H bond is broken.
- Bromine water
Bromine water is actually an aqueous form of bromine. Here in aqueous solution, phenol ionizes to form phenoxide ion due to the presence of negative charge, the oxygen of phenoxide ion donates electron on benzene ring to a large extent, as a result, the ring gets highly activated and hence tri-substituion occurs.
- Acidified KMnO4 (potassium permanganate)
Potassium permanganate is a strong oxidant and oxidizes benzyl alcohol to benzoic acid. The compound is used due to its high oxidation power and the reaction proceeds due to the presence of hydrogen attached to the carbon group (Benzylic position)
- Sulphuric acid or concentrated Phosphoric acid
Alcohols are amphoteric. So, the lone pair of oxygen atoms makes the -OH group weakly basic. Thus, in the presence of a strong acid, R—OH acts as a base and protonates into the very acidic alkyloxonium ion +OH2.This basic characteristic of alcohol necessarily helps in conversion to propene when dehydrated with a strong acid.
- LiAlH4 (lithium aluminum hydride) or NaBH4
Both compounds are best-reducing agents containing 4 Hydrogen atoms each. In reaction with a ketone, the double bond is reduced and hydrogen atoms are substituted to form alcohol.
Q 11.22 Give reason for the higher boiling point of ethanol in comparison to methoxymethane.
A 11.22
- Due to the presence of -OH group, ethanol undergoes intermolecular hydrogen bonding which results in the association of molecules.
- Therefore, extra energy is required to break those hydrogen bonds. Whereas methoxymethane does not undergo those hydrogen bonding which implies ethanol has a higher boiling point than that of methoxymethane.
Q 11.23 Give IUPAC names of the following ethers:
A 11.23
- Ethoxy-2-methylpropane
- Chloro-1-methoxyethane 4-Nitroanisole
1-Methoxypropane
- Ethoxy-4,4-dimethylcyclohexane Ethoxy benzene
Q 11.24 Write the names of reagents and equations for the preparation of the following ethers by Williamson’s synthesis: (i) 1-Propoxypropane (ii) Ethoxybenzene (iii) 2-Methoxy-2- methylpropane (iv) 1-Methoxyethane
A 11.24 During the reaction, an alkoxide ion is formed which is then added to an alkyl halide to form the ether via SN2 mechanism.
In the primary step, the nucleophile is formed (O- ) which will the approach to the alkyl halide and after the transition stage, the substitution takes place.
During the reaction, an alkoxide ion is formed which is then added to an alkyl halide to form the ether via SN2 mechanism.
During the reaction, an alkoxide ion is formed which is then added to an alkyl halide to form the ether via SN2 mechanism.
During the reaction, an alkoxide ion is formed which is then added to an alkyl halide to form the ether via SN2 mechanism.
Q 11.25 Illustrate with examples the limitations of Williamson synthesis for the preparation of certain types of ethers.
A 11.25 Williamson synthesis is basically a SN2 reaction of a primary alkyl halide with an alkoxide ion. The basic mechanism for this reaction is
Now consider this reaction,
This reaction proceeds as conventional Williamson synthesis. But if secondary or tertiary alkyl halides are taken in place of primary alkyl halides, then elimination would compete over substitution reaction, which will result in the formation of alkenes. The reason is alkoxides are better nucleophiles as well as strong bases. Therefore, they react with alkyl halides resulting in an elimination reaction.
Q 11.26 How is 1-propoxypropane synthesised from propan-1-ol? Write mechanism of this reaction.
A 11.26 According to the question we have to perform the following conversion: -
The mechanism of the above reaction is as follows : - The mechanism is given below: -
In the first step, the alcohol gets protonated by the acid present to give a protonated alcohol.
In the second step, the nucleophilic attack of another alcohol molecule on the protonated alcohol gives us 1-propoxypropane as the desired product.
Q 11.27 Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
A 11.27 The preparation of ether by acid dehydration of primary alcohol involves the nucleophilic addition of alcohol molecule to the protonated alcohol molecule as shown below: -
However, under these conditions secondary and tertiary alcohols forms alkenes rather than ethers. The reason for this being that due to stearic hindrance, nucleophilic attack by the alcohol molecule on the protonated alcohol molecule does not take place. Instead protonated 20 and 30 alcohols lose a molecule of water to form stable carbocations. The stable carbocations so formed prefers to lose a proton to form alkenes instead of forming ethers by undergoing nucleophilic attack by another alcohol molecule. This could be seen clearly from the following reactions : -
Q 11.28 Write the equation of the reaction of hydrogen iodide with: (i) 1-propoxypropane (ii) methoxybenzene and (iii) benzyl ethyl ether.
A 11.28 1. 1-propoxypropane reacts with hydrogen iodide to give propan-1-ol and 1-iodopropane as the products.
- 2. Methoxybenzene reacts with hydrogen iodide to give phenol and iodomethane
- Benzyl ethyl ether reacts with hydrogen iodide to give benzyl iodide and ethanol
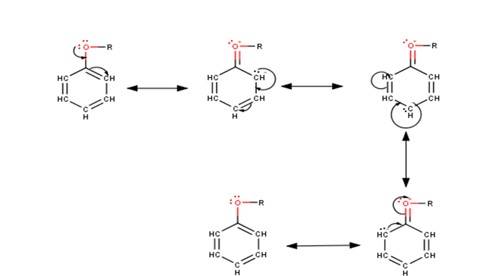
Q 11.29 Explain the fact that in aryl alkyl ethers (i) the alkoxy group activates the benzene ring towards electrophilic substitution and (ii) it directs the incoming substituents to ortho and para positions in benzene ring.
A 11.29 (i) In aryl alkyl ethers the +R effect of the alkoxy group leads to an increase in the electron density of the benzene ring as they push electrons into the ring making the benzene ring activated towards electrophilic substitution reactions. This could be understood more clearly from the following resonating structures : -
(ii) It could be clearly seen from the above resonating structures that the electron density increases more at the ortho and para positions as compared to the meta positions. Hence, we can conclude that the alkoxy group directs the incoming substituents to ortho and para positions in the benzene ring.
For example –
Q11.30 Write the mechanism of the reaction of HI with methoxymethane.
A 11.30 The reaction of HI with methoxymethane yields two different sets of products depending upon the initial amount of HI taken.
- When equal moles of HI and methoxymethane are taken, a mixture of methyl alcohol and methyl iodide is
The mechanism is given below:
In the first step, methoxymethane reacts with hydrogen iodide to extract a proton to give the dimethyloxonium ion.
In the second step of the reaction, the Dimethyloxonium ion reacts with the iodide ion present to yield methyl iodide and methyl alcohol as the product via SN2 pathway.
- If an excess of HI is used the methyl alcohol formed in Step II is also converted into methyl iodide by the following mechanism : -
In the first step, methoxymethane reacts with hydrogen iodide to extract a proton to give the dimethyloxonium ion.
In the second step of the reaction the Dimethyloxonium ion reacts with the iodide ion present to yield methyl iodide and methyl alcohol as the product via SN2 pathway.
In the third step of the reaction Methyl alcohol formed above reacts with hydrogen iodide to extract a proton to give the protonated methyl alcohol which finally reacts in the fourth step with the iodide ion via SN2 pathway to give methyl iodide and water as the product.
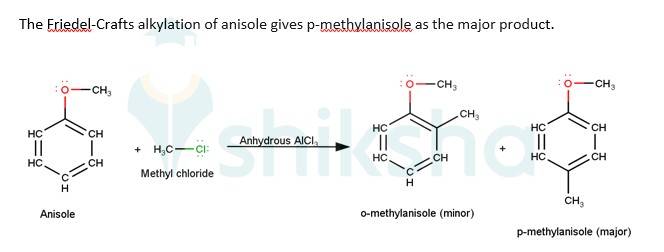
Q 11.31 Write equations of the following reactions: (i) Friedel-Crafts reaction – alkylation of anisole. (ii) Nitration of anisole. (iii) Bromination of anisole in ethanoic acid medium. (iv) Friedel-Craft’s acetylation of anisole.
A 11.31
The driving force of all the reactions given to the question is that the alkoxy group is an ortho and para directing group because it exerts its +R effect in the benzene ring. Para position being comparatively more stable than the ortho position is usually preferred because ortho position leads to stearic hindrance, hence the major product is mostly the para- substituted compound.
As seen from the resonating structures above the structure in which the negative charge is in the para position will form a more stable product when attacked by an electrophile. Hence in the following reactions, we will be considering that resonating structure as the starting material.
The mechanism is given below:
- The driving force of all the reactions given to the question is that the alkoxy group is an ortho and para directing group because it exerts its +R effect in the benzene ring. Para position being comparatively more stable than the ortho position is usually preferred because ortho position leads to stearic hindrance, hence the major product is mostly the para-substituted
-
As seen from the resonating structures above the structure in which the negative charge is in the para position will form a more stable product when attacked by an electrophile. Hence in the following reactions, we will be considering that resonating structure as the starting material.
Nitration of anisole gives p-nitroanisole as the major product.
-
The mechanism is given below:
-
- The driving force of all the reactions given to the question is that the alkoxy group is an ortho and para directing group because it exerts its +R effect in the benzene ring. Para position being comparatively more stable than the ortho position is usually preferred because ortho position leads to stearic hindrance, hence the major product is mostly the para-substituted
-
- The driving force of all the reactions given to the question is that the alkoxy group is an ortho
As seen from the resonating structures above the structure in which the negative charge is in the para position will form a more stable product when attacked by an electrophile. Hence in the following reactions, we will be considering that resonating structure as the starting material. and para directing group because it exerts its +R effect in the benzene ring. Para position being comparatively more stable than the ortho position is usually preferred because ortho position leads to stearic hindrance, hence the major product is mostly the para-substituted compound.
The Friedel-Craft’s acetylation of anisole gives 4- methoxyacetophenone as the major product.
- The driving force of all the reactions given to the question is that the alkoxy group is an ortho
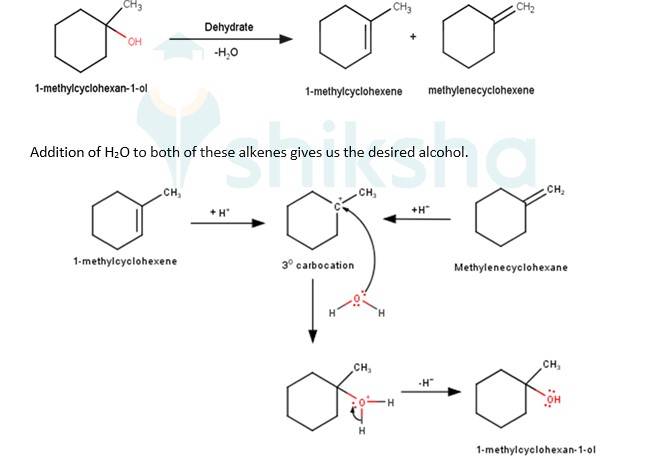
Q 11.32 Show how would you synthesise the following alcohols from appropriate alkenes?
A 11.32
- We know that the addition and elimination reactions are opposite of each other.Hence, for solving the above questions our approach should be to first dehydrate a suitable alcohol to give either a single alkene or a mixture of an alkene, if we obtain a mixture of alkene then we would have to detect which of the alkene will give us the desired alcohol. Wherever required the acid- catalyzed addition of water to alkenes will follow Markovnikov’s rule.
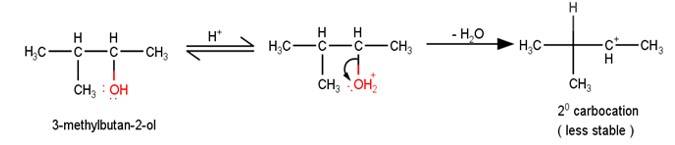
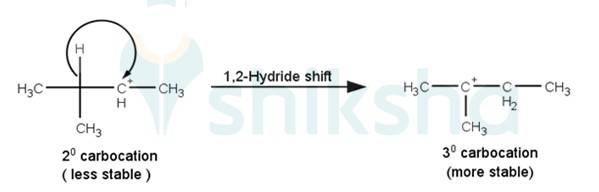
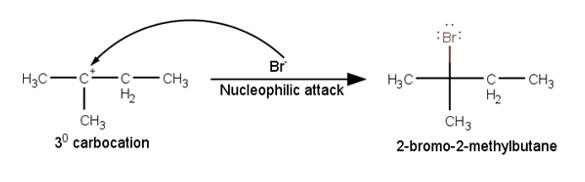
Q 11.33 When 3-methylbutan-2-ol is treated with HBr, the following reaction takes place: Give a mechanism for this reaction. (Hint : The secondary carbocation formed in step II rearranges to a more stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.
A 11.33 1. The first step in the mechanism of the given reaction is protonation of the alcohol followed by loss of water to give a 20 carbocation.
2. The next step is a rearrangement of the 20 carbocations formed in the above step is less stable it rearranges by a 1,2-hydride shift to form more stable 3° carbocations.
3. The last step of the reaction is the nucleophilic attack of Br- ion on the 3° carbocations giving the final product.
News & Updates
Chemistry Ncert Solutions Class 12th Exam

 7
7