NCERT Class 12 Surface Chemistry Solutions is available here for the reference of the students. The NCERT Class 12 Chemistry Solutions available here offer precise responses to the questions found in the NCERT textbooks. These solutions have been thoughtfully written by the subject matter experts.
Surface chemistry is a branch of chemistry that deals with the study of chemical reactions and processes that occur at the interface between two phases, typically involving a solid phase and a liquid or gas phase. It focuses on understanding the behaviour of molecules and atoms at the surface of materials and how they interact with each other and their environment.
The surface of a material, whether it is a solid, liquid, or gas, possesses distinct properties that differ from the bulk of the material. These surface properties play a crucial role in various phenomena, such as adsorption, catalysis, corrosion, and surface reactions.
Topics Covered in NCERT Chemistry Class 12 Surface Chemistry Chapter
Key concepts in surface chemistry include:
- Adsorption: Adsorption refers to the process in which molecules or atoms from a gas or liquid phase adhere to the surface of a solid or liquid. Adsorption can be physical or chemical in nature, and it is influenced by factors such as surface area, temperature, pressure, and the nature of the adsorbent and adsorbate.
- Surface reactions: Surface reactions involve chemical reactions that occur specifically at the surface of a material. The reactivity of the surface can be different from the bulk, leading to unique reaction pathways and kinetics. Surface reactions are essential in heterogeneous catalysis, where a catalyst promotes a chemical reaction by providing an active surface for the reactants to interact.
- Surface tension: Surface tension is a property of liquids that arises due to the cohesive forces between the molecules at the liquid surface. It determines the ability of a liquid to spread or form droplets on a surface. Surface tension plays a role in wetting phenomena, adhesion, and capillary action.
- Surface characterization techniques: Surface chemistry relies on various analytical techniques to study and understand the properties of surfaces. These techniques include scanning electron microscopy (SEM), atomic force microscopy (AFM), X-ray photoelectron spectroscopy (XPS), and infrared spectroscopy (IR), among others. These methods provide information about the surface composition, structure, and reactivity.
Applications of surface chemistry are widespread and include areas such as materials science, catalysis, corrosion prevention, drug delivery systems, and environmental science. By manipulating and controlling surface properties, scientists and engineers can develop new materials with enhanced performance and design more efficient chemical processes.
Overall, surface chemistry plays a vital role in our understanding of the behavior of materials at the molecular level and contributes to the development of numerous technological advancements.
NCERT Chemistry Class 12th Solution PDF - Surface Chemistry Chapter Download
Candidates can check and download here NCERT Chemistry class 12 solution for Surface Chemistry PDF. The chapter's solution is prepared by the subject experts and are easy to understand. Surface Chemistry is an important chapter and many questions are asked from this chapter in class 12 board exams as well as JEE Mains.
Download Here: NCERT Solution for Class XII Chemistry Surface Chemistry PDF
Surface Chemistry Solutions and FAQs
Get here all the questions of NCERT Class 12th Surface Chemistry Chapter.
Intext Q 5.1 Write any two characteristics of chemisorption.
A 5.1 The two characteristics of Chemisorption are:
- In Chemisorption which is highly specific in nature, the adsorb ate and adsorbent get attached by chemical bonds which are either covalent or ionic in
- High activation energy is required and high temperature is also
- Chemisorption increases with the increase in surface area which results in more number of active
Intext Q 5.2 Why does physisorption decrease with the increase of temperature?
A 5.2 As Physisorption is Exothermic in nature, which means when gas gets adsorbed on the solid surface, Heat is evolved. So, according to Le-Chatelieres when the temperature is an increased reverse process (Desorption) will be favoured. So, Physisorption decreases with the increase of temperature.
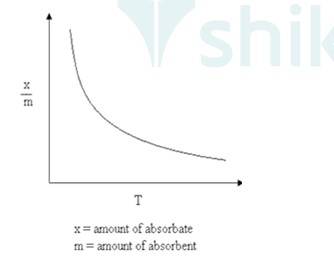
Where x/m: Volume of gas adsorbed
T: Temperature.
Intext Q 5.3 Why are powdered substances more effective adsorbents than their crystalline forms?
A 5.3 As Adsorption is directly proportional to the available surface area and powdered form of a substance have a greater surface area than the crystalline form of the substance.
So, greater the surface area of the adsorbent more is the adsorption.
Hence, powdered substances are more effective adsorbents than their crystalline forms
Intext Q 5.4 In Haber’s process, hydrogen is obtained by reacting methane with steam in presence of NiO as catalyst. The process is known as steam reforming. Why is it necessary to remove CO when ammonia is obtained by Haber’s process?
A 5.4 It is important to remove CO (Carbon Monoxide) in the synthesis of ammonia as CO affects the activity of Iron catalyst which is required in Haber’s process.
Note: Haber’s process is a very important industrial process which is used to produce ammonia.
Intext Q 5.5 Why is the ester hydrolysis slow in the beginning and becomes faster after sometime?
A 5.5 Ester hydrolysis is represented as:
Ester + Water → Acid + Alcohol
In this reaction the acid produced which is a product also acts as a catalyst and makes the reaction faster.
Such substances that act as catalysts in the same reaction in which they are obtained as products are known as Autocatalysts.
So, ester hydrolysis is slow in the beginning and becomes faster after some time as more acid is produced on the product side.
Intext Q 5.6 What is the role of desorption in the process of catalysis.
A 5.6 Desorption is a process in which substance (reactant + product) is released from the surface which is the opposite process of sorption.
The role of desorption in the process of catalysis is to make the surface of the solid catalyst-free for fresh adsorption of reactants on the solid surface for further reactions to take place.
Intext Q 5.7 What modification can you suggest in the Hardy Schulze law?
A 5.7 According to Hardy-Schulze law
‘The greater the valence of the flocculating ion added, the greater is its power to cause precipitation.
As this law takes into consideration of only the charge present on the ion and not the size of the ion. So when the size of the atom is considered, smaller the size of an atom more will be its polarising power.
So Hardy-Schulze can be modified in terms of the polarising power of the flocculating ion as ‘The greater the polarising power of the flocculating ion added, the greater is its power to cause precipitation.
Intext Q 5.8 Why is it essential to wash the precipitate with water before estimating it quantitatively?
A 5.8 The precipitate which is obtained from a chemical reaction always contain some unwanted substances (eg ions, impurities) which get adsorbed onto the surface of the precipitate.
Therefore, it becomes important to wash the precipitate before estimating it quantitatively so as to remove these unwanted adsorbed substances and obtain accurate results.
Exercise Q 5.1 Distinguish between the meaning of the terms adsorption and absorption. Give one example of each.
A 5.1 Adsorption- The accumulation of molecular species at the surface rather than in the bulk of a solid or liquid is termed as adsorption. In this substance accumulate at surface only and does not penetrate into the adsorbent (material on the surface of which adsorption takes place).
Ex- If gases like O2, H2, NH3 taken in a closed vessel containing charcoal then gases get adsorbed on the surface of charcoal and reduce the pressure.
Absorption- In this molecules get distributed uniformly throughout the material Instead of accumulating at the surface.
Ex- When gas like O2 is mixed with water then O2 get distributed throughout the solvent (Water).
Exercise Q 5.2 What is the difference between physisorption and chemisorption?
A 5.2
| Physisorption |
Chemisorption |
| 1) It happens due to Van der Waals forces. 2) It is reversible. 3) It is not specific in nature. 4) Low enthalpy of adsorption (20-40 KJ mol-1) 5) Low temperature is favourable. It decreases with increase in temperature. 6) Very less activation energy is needed. 7) It results in the multimolecular layer. |
1) It is caused by Chemical bond formation. 2) It is irreversible. 3) It is very specific in nature. 4) High enthalpy of adsorption (80-240 KJ mol-1) 5) The high temperature is favourable. It Increases with an increase in temperature. 6) High activation energy is needed. 7) It results in the unimolecular layer. |
Exercise Q 5.3 Give reason why a finely divided substance is more effective as an adsorbent.
A 5.3 Finely divided substances have large surface area compared to not divided substance. And the increase in surface area increases the adsorption. Hence Finely divided substances are more effective adsorbent.
Exercise Q 5.4 What are the factors which influence the adsorption of a gas on a solid?
A 5.4 Extent of adsorption of a gas on a solid is influenced by following factors-
- Nature of adsorbate
- Nature of adsorbent
- Pressure
- Temperature
Exercise Q 5.5 What is an adsorption isotherm? Describe Freundlich adsorption isotherm.
A 5.5 Adsorption Isotherm- The variation in the amount of a gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by the curve . This curve is known as Adsorption Isotherm.
Freundlich adsorption isotherm- Freundlich gave an empirical relationship between the quantity of gas adsorbed by a unit mass of solid adsorbent And pressure at a particular temperature.
x/m = k P1/nm
Where x is the mass of gas adsorbed on mass m of the adsorbent pressure P. k and n are constants depend on nature of adsorbent. This relationship is expressed in the form of curves where the mass of the gas adsorbed per gram of adsorbent is plotted against pressure.
Exercise Q 5.6 What do you understand by activation of adsorbent? How is it achieved?
A 5.6 Increasing the adsorbing power of adsorbent is known as activation of adsorbent.it can be achieved by
- Increase in surface area of
- By dividing the adsorbent into small
- By making its surface
Exercise Q 5.7 What role does adsorption play in heterogeneous catalysis?
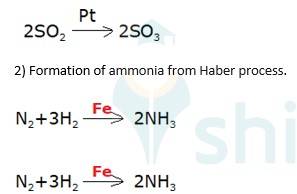
A 5.7 Adsorption of reactants on the solid surface of the catalysts increases the rate of reaction. Generally, the reactant is gas whereas catalyst is solid. Reactant molecules get adsorbed on the surface of the catalyst as a result concentration of reactant increases leads to increase in the rate of reaction. there are many important reactions is based on this, for Ex (1) manufacturing of ammonia using iron as a catalyst,
- 2. manufacture of H2SO4 By contact process,
- 3. use of finely divided Nickel in the hydrogenation of cells
Exercise Q 5.8 Why is adsorption always exothermic?
A 5.8 When adsorbate (gas or liquid mostly) absorbed then their entropy get reduced i.e. ΔS is negative. For every spontaneous process ΔG must be negative. ΔG = ΔH - TΔS, as TΔS is positive (ΔS is negative) ΔH must be negative in order to make ΔG negative. Hence ΔH is negative that means the reaction is exothermic. Hence every adsorption reactions are exothermic.
Exercise Q 5.9 How are the colloidal solutions classified on the basis of physical states of the dispersed phase and dispersion medium?
A 5.9 Colloidal solutions get classified in 8 Types on the basis of physical state of the dispersed phase and dispersion medium. This are given in following table-
| Dispersed phase |
Dispersion media |
Types of colloids |
Example |
| Solid |
Solid |
Solid Sol |
Gem stone |
| Solid |
Liquid |
sol |
Paints, cell fluid. |
| Solid |
Gas |
Aerosol |
Dust, smoke. |
| Liquid |
Solid |
Gel |
Butter, Cheese. |
| Liquid |
Liquid |
Emulsion |
Milk, Hair cream. |
| Liquid |
Gas |
Aerosol |
Fog, Mist, Cloud. |
| Gas |
Solid |
Solid Sol |
Pumice stone. |
| Gas |
Liquid |
Foam |
Froth, soap lather. |
Exercise Q 5.10 Discuss the effect of pressure and temperature on the adsorption of gases on solids.
A 5.10 Temperature- Adsorption decreases with increase of temperature because it is an exothermic process, by using le chatelier's principle the reaction will proceed in backward direction.
Pressure- Adsorption increase with increase in pressure at constant temperature.
Exercise Q 5.11 What are lyophilic and lyophobic sols? Give one example of each type. Why are hydrophobic sols easily coagulated ?
A 5.11 Lyophilic - lyophilic (liquid loving) this sols formed by direct mixing of substances like gum starch etc. with suitable liquid, called as lyophilic sols. This are called as reversible sol because if dispersion medium is separated from the dispersed phase it can be reconstructed just by mixing them. These sols are quite stable. Ex-Protein, Starch.
Lyophobic -lyophobic (liquid hating) this sols don't formed by direct mixing of dispersed phase and dispersion media they require special methods for preparation. Ex-Sols of metal sulphides . This Lyophobic(Hydrophobic-water hating) sols gets easily coagulated because they are less stable. Their stability is only due to Charge If electrolyte is added to it opposite charge will attract and form coagulation easily.
Exercise Q 5.12 What is the difference between multimolecular and macromolecular colloids? Give one example of each. How are associated colloids different from these two types of colloids?
A 5.12
| Multimolecular colloids |
Macromolecular colloids |
| 1) formed by aggregation of large number of atoms of smaller radii. |
1) Formed by particles of large size. |
| 2) Low molecular masses |
2) High molecular masses. |
| 3) Weak van der waals forces |
3) Strong van der waals Forces |
| 4) Ex-sols of gold and sulphur. |
4) Ex-Nylon, starch. |
Associated colloids- They formed by aggregation of large number of ions. instead of atoms or molecules in above two colloids. They have van der waals force directly proportional to concentration of ions. Ex- Soap Sol.
Exercise Q 5.13 What are enzymes? Write in brief the mechanism of enzyme catalysis.
A 5.13 Enzymes are complex nitrogenous organic compounds which are produced by Living plants and animals. They are protein molecules of higher molecular masses. They are effective catalysts termed as “biochemical catalysts”
Mechanism of enzyme catalysis- There are number of cavities present on the surface of colloidal particle of enzymes. They have specific shape and contains group like –COOH ,-SH, - OH. etc. they are active centers of enzyme particle molecules of reactant which have complimentary shape fit into this cavities, this forms activated complex Which then decompose to yield products. This reaction occur in 2 steps-
- Enzyme(E) + Substrate (S) → ES
- ES → Enzyme + Products
Exercise Q 5.14 How are colloids classified on the basis of (i) physical states of components (ii) nature of dispersed phase and (iii) interaction between dispersed phase and dispersion medium?
A 5.14 Colloidal solutions get classified in 8 Types on the basis of physical state of the dispersed phase and dispersion This are given in following table-
| Dispersed phase |
Dispersion media |
Types of colloids |
Example |
| Solid |
Solid |
Solid Sol |
Gem stone |
| Solid |
Liquid |
sol |
Paints,cell fluid. |
| Solid |
Gas |
Aerosol |
Dust,smoke. |
| Liquid |
Solid |
Gel |
Butter ,Cheese. |
| Liquid |
Liquid |
Emulsion |
Milk,Hair cream. |
| Liquid |
Gas |
Aerosol |
Fog,Mist,Cloud. |
| Gas |
Solid |
Solid Sol |
Pumice stone. |
| Gas |
Liquid |
Foam |
Froth,soap lather. |
2) Depending upon nature of Particles of dispersed phase it can be classified into 3 categories
- Multimolecular Colloids-In this colloidal particle consist of aggregates of atoms having diameter less than
- Macromolecular colloids-The colloids in which Large particle aggregates and dissolved into suitable
- Associated colloids – This colloids show Colloidal property at higher This contains ions of colloidal size.
3) Depending upon interactions between dispersed phase and dispersion medium it is divided into 2 categories
- Lyophilic Colloids- lyophilic (liquid loving) this colloids formed by direct mixing of substances like gum starch etc. with suitable liquid, called as lyophilic sols . This are called as reversible Colloids because if dispersion medium is separated from the dispersed phase it can be reconstructed just by mixing This sols are quite stable. Ex-Protein ,Starch.
- Lyophobic colloids- lyophobic (liquid hating) this Colloids don't formed by direct mixing of dispersed phase and dispersion media they require special methods for Ex-Sols of metal sulphides .
Exercise Q 5.15 Explain what is observed (i) when a beam of light is passed through a colloidal sol. (ii) an electrolyte, NaCl is added to hydrated ferric oxide sol. electric current is passed through a colloidal sol?
A 5.15 (i) In this case Tyndall effect is observed i.e. scattering of light by the colloidal particles takes place and the path of light become visible.
- (ii) The coagulation takes place in this case positive charge particles of Fe(OH)2 gets attached with Cl-. And of FeCl3 is obtained.
- (iii) In this case coagulation will take place. After passing electric current through colloidal solution particle will get attracted towards oppositely charged and lose their charge and coagulate.
Exercise Q 5.16 What are emulsions? What are their different types? Give example of each type.
A 5.16 Emulsions –The system which has Liquid as both dispersed phase and dispersion medium. There are two types of emulsions
- Oil in Water-Ex-Milk
- Water in oil-Ex-Butter.
Exercise Q 5.17 How do emulsifires stabilise emulsion? Name two emulsifiers.
A 5.17 Emulsifiers form an interfacial film between the suspended particles and medium. It provide coating to every drop of suspended particle and prevent it from coagulating. And it remain suspended in medium. Ex- Proteins, gums, alcohols, Lampblack etc.
Exercise Q 5.18 Action of soap is due to emulsification and micelle formation. Comment.
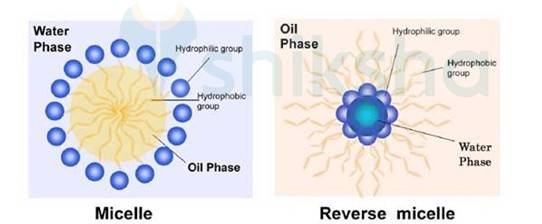
A 5.18 Soap is sodium or potassium salts of fatty acids (long chain). Represented as RCOO–Na+. When dissolved in Water it dissociates in the RCOO– and Na+. In first ion there are two parts R and COO–. R is hydrophobic tail and COO– Hydrophilic head. RCOO– ions present at the surface with COO– group inside and R group outside. Increasing concentration to critical micelle concentration anions are pulled inside the bulk and Form a spherical shape of hydrocarbon chain pointing towards the centre and COO– pointing outside this are called as micelle.
Cleansing action of soap: Soap molecules form micelle around oil droplets. Soaps emulsifies the oil or grease droplets and form emulsion after washing the cloth this emulsion easily gets removed. Hence Action of soap is due to emulsification and micelle formation.
Exercise Q 5.19 Give four examples of heterogeneous catalysis.
A 5.19 Oxidation of sulphur dioxide into sulphur trioxide in the presence of
(3) Oxidation of ammonia into nitric acid in presence of platinum gauze in Ostwald's process
→ 4NH3(g)+ 5O2(g) Pt(s) 4NO +6H2O
(4) Hydrogenation of vegetable oils in presence of Nickel as catalyst
Vegetable oil(l)+H2(g) Ni → Vegetable ghee (s)
Exercise Q 5.20 What do you mean by activity and selectivity of catalysts?
A 5.20 Activity - It is the ability of the catalyst to accelerate the Reaction. It mostly depends upon the Chemisorption strength.
Selectivity - It is an ability to direct reaction to yield of a particular product i.e., One catalyst cannot be a catalyst for other reactions.
Exercise Q 5.21 Describe some features of catalysis by zeolites.
A 5.21 Catalysis by zeolites is dependent on shape. Because zeolites are shape-selective catalysts. They are alumino silicates which are microporous in nature. It has Honeycomb structure. That makes them shape selective. In zeolites, some si atoms are replaced by Al to form Al-O-Si network.
Reactants are very sensitive to the pore size of zeolites. Zeolites are used in petrochemical industry. Ex- ZSM-5 used to convert alcohol into gasoline.
Exercise Q 5.22 What is shape selective catalysis?
A 5.22 The catalytic reaction that depends upon the pore structures of the catalysts and the size of reactant and product molecules is called shape selective catalysis. Zeolites are good shape- selective catalysts.
Exercise Q 5.23 Explain the following terms: Electrophoresis Coagulation Dialysis Tyndall effect
A 5.23
- Electrophoresis: The movement of colloidal particles under an applied electric potential is called When an electric potential is applied to two platinum electrodes dipping in colloidal solutions the colloidal particle move towards the Oppositely charged electrodes.
- Coagulation: The process of settling of colloidal particles is called coagulation. When the charge is removed from colloidal solution somehow, then particle start coagulation and settling due to the force of
- Dialysis: It is a process of removing a dissolved substance from ac colloidal solution by means of diffusion through a suitable the animal membrane or parchment paper used as a membrane.
- Tyndall Effect: When homogenous solution is placed in a dark and viewed in light beam then if it is viewed from sides it appears visible but if it is viewed from 90° it appears to be dark. This effect is known as Tyndall
Exercise Q 5.24 Give four uses of emulsions.
A 5.24 Uses of Emulsions-
- It is used in making of medicines,
- Cleansing action of soaps is based on this emulsion
- Digestion of fats in intestine takes place by the process of
- Antiseptics and disinfectant added to water form emulsion for
Exercise Q 5.25 What are micelles? Give an example of a micelles system.
A 5.25 Soaps contain hydrophobic and hydrophilic part when dissolved in the water they arrange themselves in such a way that they form a spherical structure having hydrophobic part towards the centre and hydrophilic part away from centre. This cluster is known as Micelle. Ex-Sodium stearate + Water
(CH3 (CH2)16COO-Na + H2O)
Exercise Q 5.26 Explain the terms with suitable examples: (i) Alcosol (ii) Aerosol (iii) Hydrosol.
A 5.26 Alcohol- a colloidal solution having alcohol as the dispersion medium and a solid substance as the dispersed Ex- colloidal sol of cellulose nitrate in ethyl alcohol.
- Aerosol- a colloidal solution having gas as the dispersion medium and a solid substance as the dispersed Ex-Smoke.
- Hydrosol- a colloidal solution having Water as the dispersion medium and a solid substance as the dispersed Ex-Gold sol.
Exercise Q 5.27 Comment on the statement that “colloid is not a substance but a state of substance”.
A 5.27 We can say that colloid is not a substance but a state of a substance which is dependent on the size of particle colloidal state is intermediate between a true solution and a suspension. When a size of substance is between 1nm to1000nm it behaves as colloid otherwise not.
News & Updates
Chemistry Ncert Solutions Class 12th Exam