- Introduction
- Difference between Nitrate and Nitrite
- Nitrate and Nitrite in Class 12
- FAQs on Nitrate and Nitrite
Introduction
Nitrogen is one of the most prevalent elements in living organisms apart from carbon, hydrogen, and oxygen. It is present in amino acids, proteins, hormones, chlorophylls, and various vitamins. The nitrogen present in the atmosphere undergoes a biogeochemical cycle known as the nitrogen cycle to convert into multiple chemical forms.
Nitrates and nitrites are inorganic compounds found in the nitrogen cycle. They are most commonly detected in groundwater, soils, vegetables, and meat products. They also naturally occur in volcanic and igneous rocks.
Nitrate is more stable than nitrite, and hence, bacteria present in soils often convert nitrite into nitrate. However, when there is a lack of oxygen, other bacterias convert the nitrate into nitrite.
Difference between Nitrate and Nitrite
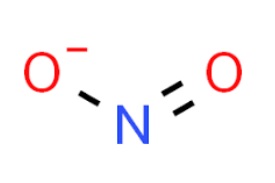
The terms nitrates and nitrites are often used interchangeably due to their similar names and spellings. However, they are two completely different compounds with different shapes, structures, and functions. The most prevalent out of all the distinguishing factors between the two compounds is their respective structures.
-
Structure of Nitrate ion
Nitrate and Nitrite in Class 12
The chapter on p-block elements in class 12 includes a complete and detailed explanation of nitrogen. The chapter covers the oxides of nitrogen, nitrogen reactions with other elements, its structure, and uses. It also discusses the structure, functions, uses, and reactions of nitrates and nitrites with other elements.
FAQs on Nitrate and Nitrite
Q. Which is worse for the human body, nitrate or nitrite?
Q. Are nitrate and nitrite basic?
Q. What are the effects of nitrate and nitrite on children?
Q. Are nitrate and nitrite soluble in water?
Q. Can nitrate and nitrite cause cancer in human bodies?
Chemistry The p-Block Elements Exam
Student Forum
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test