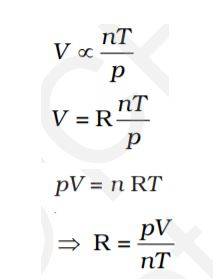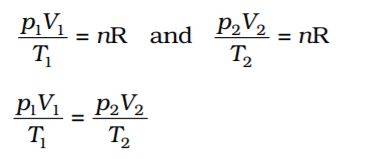- Ideal Gas Law
- The Gas Laws
- Boyle's Law
- Charles's Law
- Avogadro Law
- Ideal Gas Equation
- Ideal Gas Law for Class 11
- Illustrative Examples
- 1. What is the pressure required to compress 500 dm3 of air at 1 bar to 200 dm3 at 30°C?
- FAQ’s
Ideal Gas Law
As a result of experimental studies, the gas laws are relationships between the measurable properties of gases like Pressure, Temperature, Volume, and Mass. The relationship between these variables can describe the state of a gas.
The Gas Laws
The research on the physical properties of gases leads to the discovery of Gas Laws. There are three gas laws; Boyle's Law, Charles's law, and Avogadro's Law. A gas that follows all three laws is ideal.
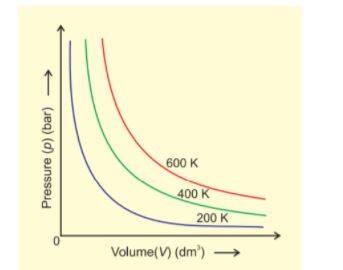
Boyle's Law
At a constant temperature, the pressure of a fixed amount of gas (number of moles, n) varies inversely with its volume. At constant t and n,
p ∝ 1/V
p = K₁ 1/V
K is a proportionality constant. At a constant temperature, the product of volume and pressure of a fixed amount of gas is constant.
K = pV
Reference: NCERT
The graph shows that as the pressure decreases, volume increases.
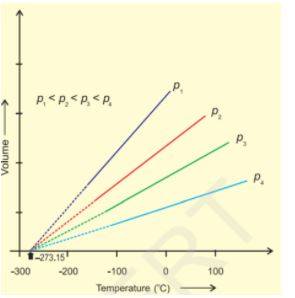
Charles's Law
At a constant pressure, the volume of a fixed mass of gas will be directly proportional to its absolute temperature.
V ∝ T
V = KT
K =V/T
The constant value is determined by the pressure of a gas, its amount, and the volume.
Reference: NCERT
The chart shows that as the Volume increases, Temperature increases.
Avogadro Law
The Law states that equal volumes of gases contain an equal number of molecules under identical temperature and pressure conditions.
V ∝ n, n is the number of moles.
V =K n
For one mole of a gas, the number of molecules present is 6.022× 10²³. It is known as the Avogadro constant.
Ideal Gas Equation
The three laws combine in a single equation is known as the Ideal gas equation.
At constant T and n; p ∝ 1/V
At constant p and n; V ∝ T
At constant p and T; V ∝ n
Thus,
Reference: NCERT
The above equation represents an Ideal gas equation. R’s value is the same for all gases, and the numerical value of R depends upon units in which p, V and T are measured. So, R is a Universal Gas constant.
R = 8.3145 Joules · mol-1 · K-1 (SI Unit) = 0.082057 L · atm·K-1 · mol-1
Ideal Gas Law for Class 11
The chapter explains the laws governing the behaviour of ideal and real gases. It also deals with how to apply gas laws in different situations. This topic's weightage is 5 marks, and the questions on gas laws expressions and graphs, Ideal gas Equation, and numerical on gas laws.
Illustrative Examples
1. What is the pressure required to compress 500 dm3 of air at 1 bar to 200 dm3 at 30°C?
FAQ’s
Physics Laws of Physics Concepts Exam
Student Forum
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test