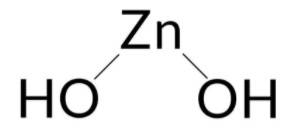
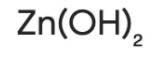
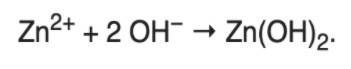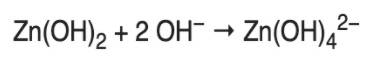Zinc Hydroxide is an inorganic chemical compound (jelly-like), made up of three rare minerals i.e. Wulfingnite (orthorhombic), Ashoverite, and Sweetite (both tetragonal). These minerals occur naturally and are the natural polymorphs of Zinc Hydroxide [Zn (OH)2]. Zinc hydroxide reacts well with both acids and bases. It is an insoluble hydroxide that reacts with strong acid and gets broken down or dissolved.
- Chemical Properties of Zinc Hydroxide
- Physical properties of Zinc Hydroxide
- Preparation/Structure of Zinc Hydroxide:
- Applications/Uses of Zinc Hydroxide:
- FAQs on Zinc Hydroxide
Chemical Properties of Zinc Hydroxide
| Chemical Formula |
Zn (OH)2 |
| Molar mass |
99.424 g/mol |
| Density |
3.053 g/cm³ |
| Melting Point |
125° C (257° F) |
Physical properties of Zinc Hydroxide
| Valency |
2 |
| Ph level |
8.88 |
| Solubility product |
3.0 × 10^-17 |
| Solubility in alcohol |
Insoluble |
Preparation/Structure of Zinc Hydroxide:
Zinc Hydroxide can be set up by adding sodium hydroxide mix, not in abundance. Then, a transparent white precipitate will form:
Applications/Uses of Zinc Hydroxide:
- It is widely used in the preparation of medicines or surgical dressings as it functions as an absorbent. Bandages that are used after surgeries are coated with the zinc substance to absorb the blood from the wound.
- Galvanization is the process in which steel and iron are coated with a layer of zinc and it is highly used in this process. Zinc prevents steel and iron from getting rusted.
- It is also used for electrical rechargeable batteries.
- It is regularly utilized as a medium for the business production of pesticides and their pigments.
- Oddly it is also used as a compounding agent between different ingredients of rubber.
- It also can be used as a mordant which means that it is used to set the dyes of fabrics or tissues.
As per the breakdown of marks for the ongoing year 2021, This chapter of Acids, bases & salts holds a weightage of 4 marks.
FAQs on Zinc Hydroxide
Q: What is the Ph level of the Zinc Hydroxide?
Q: Name two minerals of which Zinc Hydroxide is made of
Q: What is the chemical formula for Zinc Hydroxide?
Q: At what temperature does Zinc Hydroxide start to melt?
Q: Explain one major use of Zinc Hydroxide in the medical field.
Chemistry Acids, Bases and Salts Exam
Student Forum
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test