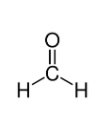
With the formula CH2O (H−CHO), formaldehyde is a naturally occurring organic compound. The pure compound is a colourless gas that naturally polymerises into paraformaldehyde, so it is stored as an aqueous solution (formalin). It is the simplest of the (R-CHO) aldehydes. This substance's common name derives from its resemblance and relation to formic acid.
Being the simplest aldehyde, formaldehyde has a very basic structure. The molecule consists of a carbon atom from which a single bond attaches two hydrogen atoms and an oxygen atom from which a double bond binds to the carbon atom. The following points include a detailed chemical and structural formula for formaldehyde (methanal).
Formaldehyde Chemical Formula
The formaldehyde chemical formula is given as— CH2O. The molar mass of formaldehyde is of the value 30.026 g/mL.
Structural formula
Formaldehyde formula in Class 10
In the chapter Carbon and its compound, you will get to learn about some basic concepts. For essence, how it is named, and what happens when one of the H is changed by an alkyl group. The weightage is 5 marks.
Formaldehyde formula in Class 11
In the GOC part of organic chemistry, you are going to learn about the structure, hybridisation, naming convention in detail.
Formaldehyde formula in Class 12
Every chapter of Class 12, insist more on the application part. So, under chapter Aldehyde, Ketones and Carboxylic Acids, you will get to learn about the formaldehyde and different reactions in which formaldehyde and the other members of the aldehyde family undergo. The chapter is a part of 28 marks.
Illustrated Examples
1. What is formaldehyde as a functional group?
Answer: The formaldehyde molecule has an aldehyde H-C=O with the normal functional group. It is bound to a hydrogen, such that the simplest aldehyde is formaldehyde. The C atom has sp2 hybridisation, so the molecule's geometry is planar-trigonal.
2. Is formaldehyde basic or acidic?
Answer: Like other aldehydes, formaldehyde, also from the air, absorbs oxygen relatively readily and is therefore oxidised to formic acid. Formaldehyde solutions arrive reasonably easily and retain a pH of 3.5 or even 3.
3. What is formaldehyde used in?
Answer: Formaldehyde is a strong-smelling, colourless gas used in the manufacturing of construction materials and many household items. It is used in items made of pressed wood, such as particleboard, plywood, and fiberboard; glues and adhesives; garments made of the permanent press; coatings of paper products; and some insulation materials.
[Image courtesy: NCERT]
FAQs on Formaldehyde Formula
Q: How does the human body do with formaldehyde?
Q: Is formaldehyde a disinfectant?
Q: Are formaldehyde and formalin the same?
Q: What are the disadvantages of formaldehyde?
Q: Is formaldehyde explosive?
News & Updates
Aldehydes, Ketones and Carboxylic Acids Exam
Student Forum
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test