- Quantum Numbers
- Types of Quantum Numbers
- Quantum Numbers for Class 11
Quantum Numbers
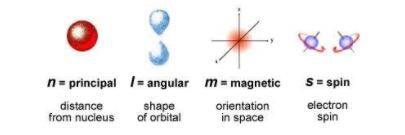
The quantum numbers determine the movement of an electron in an atom. They are used to identify the position and energy of an atom. The sum of all the quantum numbers in all the atoms must comply with the Schrodinger equation. The quantum numbers are classified into four main headings:
- Principal quantum numbers
- Azimuthal quantum numbers
- Magnetic quantum numbers
- Spin quantum numbers
These four quantum numbers can identify all the attributes of an atom in a detailed manner. The symbols and possible values of all the quantum numbers can be seen below:
| Number | Symbol | Possible values |
|---|---|---|
| Principal quantum number |
n |
1,2,3,4…. |
| Angular momentum quantum number |
l |
0,1,2, 3…, (n-1) |
| Magnetic quantum number |
m1 |
-l…. -1,0,1…., l |
| Pin quantum number |
ms |
+1/2, -1/2 |
Types of Quantum Numbers
As discussed above, there are four types of quantum numbers; let us study them in more detail.
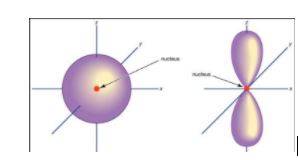
1. Principal quantum number – The symbol of principal quantum number is ‘n.’ They are defined as the main electron shell in an atom. It is used to calculate the difference between the nucleus and the electron in an atom. The larger the distance, the larger will be the principal quantum number.
The innermost shell of an electron can be denoted as n=1.
2. Azimuthal quantum number – The shape of an orbital can be determined by an azimuthal quantum number. The symbol ‘l’ is used for the azimuthal quantum number. The value of the sum of angular nodes is equal to the azimuthal quantum number. The range of azimuthal quantum numbers lies between 0 and (n-1).
3. Magnetic quantum number – The orbitals make their subshells, and the total number of these orbitals and orientation is determined by the magnetic quantum number. The orbitals correspond with the projected amount of the angular movement. Let’s take a closer look at the subshells of the magnetic quantum number.
| An azimuthal quantum number value |
Number of orbitals (2l +1) |
Possible values |
|---|---|---|
| 0 (s subshell) |
2*0 + 1 = 1 |
0 |
| 1 (p subshell) |
2*1 + 1 = 3 |
-1, 0, and 1 |
| 2 (d subshell) |
2*2 + 1 = 5 |
-2, -1, 0, 1, and 2 |
| 3 (f subshell) |
2*3 + 1 = 7 |
-3, -2, -1, 0, 1, 2, and 3 |
4. Electron spin quantum number – The symbol of electron spin quantum number is ms. It is used to determine the position in which the electron is spinning. The positive value indicates the upward spin and the negative value indicates the downward spin.
Quantum Numbers for Class 11
The chapter ‘Chemical Bonding and Molecular Structure’ holds a weightage of 5 marks in total. It consists of one short and one objective type of question.
Illustrated Examples
- Illustrate the principal quantum number.
Sol:
Source-ncert
- Illustrate an understanding of the 4 quantum numbers.
Sol:
Source-ncert
- Explain the possible outcome of the values from the angular momentum quantum number.
Following are the possible values that are expected as an outcome for AMQN –
0, 1, 2, 3…, (n-1).
FAQs
Q: What’s the origin of the principal quantum number?
A: Bohr’s model.
Q: What do you understand by the spin of an electron?
A: The spin of an electron is one of the main properties of an electron. The rotation of an electron around its axis is referred to as the spin of the electron.
Q: Explain the reason behind atoms having only 8 electrons in the outer shell.
A: The concept of an atom having 9 electrons in the outermost shell derives from the stability that noble gases possess.
Q: What are the classifications of quantum numbers?
A: Principal quantum numbers, Azimuthal quantum numbers, Magnetic quantum numbers, and Spin quantum numbers.
Q: What’s the symbol for magnetic QN?
A: M1
Physics Laws of Physics Concepts Exam
Student Forum
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test