Van Der Waals Forces
In this topic, we are going to learn about Van Der Waals Forces, intermolecular forces. Van Der Waals Forces can be defined as the pull of attraction caused by bonding in molecules or atoms. These interactions are intended towards molecules lying without charge because the electrostatic forces within the range start acting up. These are also termed as the weakest molecular range consisting of the dipole to dipole dispersion. The main cause for these forces to occur resides behind the fluctuation that takes place in the polarization of at least two particles located at a very near distance.
These Van Der Waals Forces are considered the weakest chemical forces because as soon the distance between two particles starts increasing at a molecular interaction level, the intermolecular forces quickly vanish. It should be noted that these electrostatic forces attract uncharged molecules towards each other but in all states or forms – solids, liquids, and gas.
The equation for Van Der Waals Forces
In the below-mentioned equation, one can observe the main two kinds of properties present – the first being the volume of both the elements and the second being the attractive forces in-between. The equation is as follows:
Van Der Waals forces and their characteristics
- Van Der Waals Forces are significantly weaker as they are electrostatic, but covalent and ionic bonds are much stronger than Van Der Waals Forces.
- Saturation of Van Der Waals Forces isn’t possible.
- Van Der Waals Forces doesn’t have any attributed sense of direction.
- These forces do not depend upon temperature.
Types of Van Der Waals Forces
Keesom Interactions
These interactions are electrostatic and occur because of the charges present in the ionic molecules. This type is named after a Ditch physicist Willem Hendrick Keesom. These are temporary interactions and only take place between two permanently existing dipoles.
Debye Forces
These are the causes of induced dipoles, which take place between permanent dipoles and some of the other atoms. Unlike Keesom, this takes place between permanent and no temporary dipoles.
London Dispersion Forces
These interactions are caused because the main reason behind these is the instantaneous dipole attraction towards another molecule. These are the weakest type of Van Der Waals Forces.
Van der Waals Forces for Class 11
As per the latest published CBSE syntax for marks and its breakdown, this chapter of States of Matter holds a weightage of 5 marks in total. It contains 1 very short question of 2 marks each and one short question for 3 marks each. This brings the overall weightage of this chapter to 5 marks in total.
Illustrated Examples
1. Provide an example of London Dispersion forces.
Answer:
FAQs on Van Der Waals Forces
Q: Name one factor which affects the strength of Van Der Waals Forces.
Q: Explain saturation in Van Der Waals Forces.
Q: Does Van Der Waals Forces depend upon temperature?
Q: Which is the weakest type of Van Der Waals Forces.
Q: Name one type of Van Der Waals Forces.
News & Updates
States of Matter Exam
Student Forum
Answered 2 months ago
Yes if you don't got the seat it is refundable and In case you got the seat it will adjusted in your admission fees
D
Beginner-Level 1
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test

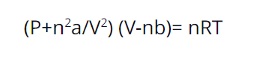


is the extra 86k that is required for mop up round of mp state counselling for MBBS refundable?