
- Monomeric Proteins
- What Are the Monomers of Protein Bonds?
- Composition and Structure of Protein
- Monomers of Proteins
- Monomeric Proteins in Class 11
- Monomeric Proteins in Class 12
- FAQs on Monomeric Proteins
Monomeric Proteins
Monomers are small molecules present in compounds. They are mostly organic but can also be synthetic. The easy availability of cheap chemicals and diversifications during industrialization led to an increased interest in monomers and the rise of the plastic age.
What Are the Monomers of Protein Bonds?
Every living organism on this planet has cells that consist of several molecules like nucleic acids, polysaccharides, and proteins. These large molecules are made up of smaller structures called monomers. These monomers combine to form polymers through a process called polymerisation.
Amino acids polymerise to form proteins. Amino acids contain hydrogen, amine, R-group, and the carboxyl group. These elements form various bonds covalently to produce proteins. These bonds are called peptide bonds.
Scientists are now able to produce similar protein-like polymers through the polymerisation of amino acids under controlled conditions. Similar abiotic polymerisation of nucleotides and sugars is also possible and has helped us develop various biomolecules. Owing to the highly polar and reactive protein structure, we can also make different biopolymers using polymeric materials.
Composition and Structure of Protein
Carbon, hydrogen, oxygen, nitrogen, and sulfur form covalent bonds to produce proteins. Globular proteins occur when about one-third of residues turn in loops and reverse the polypeptide chain. These loops are known as the third type of ordered secondary structure.
Apart from this, there are four main structures of proteins:
- Primary structure: Protein consists of both amino acid sequence and hydrogen bonds. The amino acid sequence makes up the primary structure of a protein molecule. The hydrogen bonds play a role in determining the secondary structure of the protein.
- Secondary structure: The alpha-helix structure of a protein is its secondary structure. In this structure, a single protein chain forms a coiled spring-like shape. The hydrogen bonds maintain this coil-like structure.
- Tertiary structure: A secondary folding can occur due to the peptide bond interaction with sulfur atoms present in some proteins. This interaction can change the structure and affect the function of the proteins.
- Quaternary structure: The spatial distance and relationship between the protein's amino acids will determine the quaternary structure.
Monomers of Proteins
There are about 20 kinds of amino acids that serve as building blocks for the protein.
Non-essential amino acids are acids that can be synthesised in the body. The amino acids which cannot be synthesised in the body are called essential amino acids. A balanced diet can help in the procurement of essential amino acids for the body.
These amino acids are further classified into hydrophobic, hydrophilic, and neutral categories.
The names of these 20 amino acids are as follows:
- Valine (Val)
- Leucine (Leu)
- Isoleucine (Ile)
- Methionine (Met)
- Phenylalanine (Phe)
- Cysteine (Cys)
- Asparagine (Asn)
- Glutamic acid (Glu)
- Glutamine (Gln)
- Histidine (His)
- Lysine (Lys)
- Arginine (Arg)
- Aspartic acid (Asp)
- Glycine (Gly)
- Alanine (Ala)
- Serine (Ser)
- Threonine (Thr)
- Tyrosine (Tyr)
- Tryptophan (Trp)
- Proline (Pro)
Monomeric Proteins in Class 11
The chapter Biomolecules in class 11 defines and states the uses of monomers and polymers. The process of polymerisation is also thoroughly explained in it.
Monomeric Proteins in Class 12
The chapter Biomolecules in class 12 defines and explains the structure and functions of proteins and amino acids.
[Image courtesy: NCERT]
FAQs on Monomeric Proteins
Q : What are the common types of secondary structure of proteins?
A: There are two common types of secondary structure of proteins:
- α–helix structure
- β–pleated sheet structure
α–Helix structure
If the size of R-groups is quite large then the intramolecular bonds are formed between the C=O of one amino acid and the N-H group of the forth amino acid residue in the chain. This causes the polypeptide chain to coil up into a spiral structure called righ handed α-helix structure.
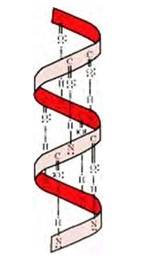
β -pleated sheet structure
In this conformation, the polypeptide chains lie side by side in a zig0zag manner with alternate R groups on the same side situated at fixed distances apart. The two such neighbouring polypeptide chains are held together by interbounded to form a sheet. These sheets are then stacked one above the other like the pages of the book to form a 3-D structure. This structure resembles pleated folds of drapery and hence is called β–pleated sheet structure. The polypeptide chains can link together in parallel and anti- parallel sequence. Such sheet like structure can easily slip on each other. Proteins of this structure are soft.
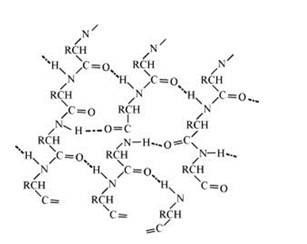
Q: What type of bonding helps in stabilising the α-helix structure of proteins?
A:The stability of α-helix structure is due to intramolecular hydrogen bonding between –NH and –CO groups of polypeptide chain. The α-helix is known as 3.613 because each turn of α-helix contains approximately 3.6 amino acids and a 13-membered ring is formed by hydrogen bonding.
Q: Differentiate between globular and fibrous proteins.
A:
| Globular proteins |
Fibrous proteins |
| It is usually soluble in water. |
It is insoluble in water. |
| All enzymes are globular protein. Some hormones such as insulin are also globular protein. |
Fibrous proteins are usually used for structural purposes. For example, keratin is present in nails and hair; collagen in tendons; and myosin in muscles. |
| The polypeptide chain in this protein is folded around itself, giving rise to spherical structure. |
It is a fibre-like-structure formed by the polypeptide chain. These proteins are held together by strong and disulphide bonds. |
| They are soluble in acids and bases. |
They are insoluble in acids and bases. |
| Examples:- egg albumin, casein of milk. |
Examples :- Silk, Skin, Wool. |
| They have weak intermolecular hydrogen bonding. |
They have comparatively stronger intermolecular forces of attraction. |
| They have folded, ball like structure. |
They have thread like structure. |
| Globular proteins are also called as spheroproteins owing to their shape. |
Fibrous proteins are also called as scleroproteins. |
| More sensitive to the changes in pH, temperature etc. |
Less sensitive to the changes in pH , temperature etc. |
Q: Do proteins have many functions in our body?
A: Yes, proteins occur in every part of the body and are required for the maintenance and growth of the body.
Q: What are the different types of monomers?
A: Sugar, amino acids, fatty acids, and nucleotides are the different monomers found in the body.
Q: What are the different types of proteins?
A: Proteins can be classified into fibrous proteins and globular proteins based on their shape.
Q: Who discovered monomers?
A: German chemist Hans von Pechmann discovered monomers.
Q: How are amino acids classified?
A: Amino acids are classified into basic, acidic, or neutral based on the amino acids and carboxyl groups present in the molecules.
Chemistry Biomolecules Exam
Student Forum
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test

