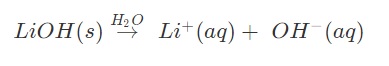The Arrhenius idea, a substance with hydrogen particles and might without issues give hydrogen particles of protons in its watery arrangement, is known as Arrhenius corrosive. For instance, while hydrochloric corrosive shifts in the water, it structures chloride particle (Cl–) and hydronium particles (H₃O⁺). Essentially, acidic corrosive (CH3COOH) likewise goes about as an Arrhenius corrosive in its watery answer due to its administration of acetic acid derivation particle (CH3COO–) and the hydronium particle.
Basicity of Arrhenius Acid
Basicity of an Arrhenius acid is termed because of the number of replaceable hydrogen ions found in an acid. As an instance, Phosphoric acid (H3PO4) ionises in its aqueous answer and offers three hydrogen ions; thus, phosphoric acid's basicity is three.
H3PO4 → H+ + H2PO4–
H3PO4– → H+ + HPO42-
HPO42- → H+ + PO43-
some different examples are as follows:
HCl , HNO3 , HClO4 , H3PO2 , H3BO3 (Monobasic acid (basicity = 1))
(COOH)2, H2SO4, H2SO3, H3PO3 (Dibasic acid (Basicity = 2)
H3PO4 (Tribasic acid (Basicity = 3)
Strong Electrolytes
Arrhenius, who became seeking to find out why certain solutions ought to conduct an electric contemporary, discovered that conductivity arose from the presence of ions. He discovered that when the substances HCl, HNO3, and H2SO4 were dissolved in water, they behave as robust electrolytes. This turned into the result of ionisation reactions in water.
As these substances are sturdy electrolytes that produce H+ ions, they are called sturdy acids. This was the result of ionisation reactions in water.
- Strong acid
Robust acids get completely ionised in an aqueous answer and grow the protons (H+) inside the solution.
HA ↔ H+ + A–
The equilibrium constant for the dissociation of acid is known as acid dissociation regular (Ka). The significance of Ka may be very excessive for strong acids. For this reason, the strength of an acid is at once proportional to the acid dissociation regular (Ka).
- Weak Acid
Because of their partial or incomplete dissociation, Susceptible acids do not launch hydrogen ions and, for that reason, exist as an equilibrium aggregate of undissociated acid in conjunction with the ions released for the duration of the partial dissociation. The range of hydrogen ions is shallow, and they display an excessive pH fee compared to strong acids. The acid dissociation constant is less for vulnerable acids in comparison to strong acids.
In Class 11: In the category of chemistry, in chapter Equilibrium, Arrhenius acid has been discussed thoroughly, with the detailed chapter and reactions, where the chapter has the weightage of 20 Marks.
Illustrated Examples
1. Explain the Arrhenius reaction of LiOH
Answer:
FAQs on Arrhenius Acid
Q: Give some examples of Arrhenius acids.
Q: What are some limitations of the Arrhenius study?
Q: Which compound is acid released when it dissolves in water?
Q: Which element is always present in Arrhenius acid?
Q: In the equation: HF + H2O H3O+ + F-, what HF and F-?
News & Updates
Chemical Equilibrium Exam
Student Forum
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test