Introduction
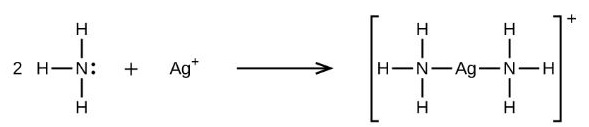
Lewis acids and bases are depicted by the Lewis hypothesis of corrosive base responses as electron-pair acceptors and electron pair contributors individually. Along these lines, a Lewis base can give a couple of electrons to a Lewis corrosive to shape an item containing a facilitated covalent bond. This item is likewise alluded to as a Lewis adduct. A delineation specifying the response between a Lewis corrosive and base prompting the arrangement of an organised covalent connection between them is given below.
Lewis Acids
Lewis Acids are the synthetic species that have void orbitals and can acknowledge electron sets from Lewis bases. This term was traditionally used to depict compound species with a three-sided planar structure and a vacant p-orbital. An illustration of such a Lewis corrosive would be BR3 (where R can be a halide or a natural substituent).
Example of Lewis Acids:-
- Cations of metals, for example, Mg2+ and Li+, can frame coordination mixes with water going about as the ligand. These aqua edifices can acknowledge electron combines and act as Lewis acids.
- Carbocations given by H3C+ and other three-sided planar species will, in general, acknowledge electron sets.
As discussed above, they are examples of the acceptors of electron pairs. Any other chemical compounds that are electron deficient π systems can act as an acceptor of electron pairs—for instance, enones.
Lewis Bases
The most widely recognised Lewis bases are smelling salts, alkyl amines, and other traditional amines. Usually, Lewis bases are anionic in nature and their base strength, by and large, relies upon the pKa of the relating guardian corrosive. Since Lewis bases are electron-rich species that can give electron-sets, they can be delegated nucleophiles. Additionally, Lewis acids can be named electrophiles (since they carry on as electron-pair acceptors).
Examples of Lewis Bases:-
- Pyridine and the subordinates of pyridine can go about as electron pair benefactors. In this manner, these mixes can be named Lewis bases.
- The mixes in which oxygen, sulfur, selenium, and tellurium (which have a place with bunch 16 of the Periodic Table) display an oxidation condition of - 2 are, for the most part, Lewis bases. Instances of such mixes incorporate water and ketones.
Weak Lewis acids have a strong lewis base (conjugately). Moreover, some chemicals with the lone pair of electrons such as CH3– and OH–are termed as Lewis bases as they can donate the electrons.
Lewis Acids and Bases in Class 11
In the chapter ‘Equilibrium’, the topic lewis acids and the base have been discussed where the properties, uses, and reactions are explained, as mentioned above.
The chapter Equilibrium contains the weightage of 20 Marks.
Illustrated Examples
1. The reaction of Lewis acids and bases with H+ ions.
Answer: The reaction between the water molecule and the protons tends to make a hydronium ion (H3O+)
FAQs on Lewis Acids and Bases
Q: Can hydrochloric acid be classified as a Lewis acid?
Q: How can ions such as Li+ and Mg2+ accept pairs of electrons?
Q: What are some examples of Lewis bases?
- Amines with the general formula R-NH3, such as methylamine.
- The fluoride ion (F–)
- Ammonia (NH3)
Q: Can we consider acetate a Lewis base?
Q: How can we consider an element as a Lewis acid?
News & Updates
Chemical Equilibrium Exam
Student Forum
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test