Carbon is known to frame various mixes because of the unique properties it conveys with itself. The broadest or the fundamental compound shaped via carbon is methane (CH4). Such sorts of combinations shaped by the mix of hydrogen and carbon are known as hydrocarbons. You can undoubtedly figure the sub-atomic equation of such kinds of compounds by merely adding hydrogen to fulfil carbon molecules' valency.
Types of Saturated Compounds
Saturated Carbon Compounds
These are the mixes in which single bonds connect different carbon molecules in a chain or a ring as they were. Alkanes are the most widely recognised instances of soaked chain carbon mixes. Ethane is an individual from the alkane family whose structure is drawn beneath:
Unsaturated Carbon Compounds
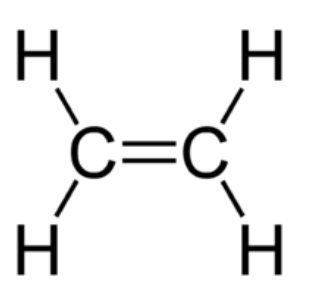
These are the mixes in which twofold or triple bonds connect different carbon particles in a chain or a ring. Alkenes (where carbon particles are connected through twofold securities) and alkynes (where carbon iotas are connected through triple securities) are the most widely recognised instances of unsaturated chain carbon mixes. Ethene is an individual from the alkene family whose structure is drawn underneath:
Properties
- Perhaps the most remarkable properties of carbon are its capacity to make long carbon chains and rings. This property of carbon is known as catenation.
- Carbon has numerous exceptional capacities out of every kind capacity because carbon structures pπ-pπ bonds that are only twofold or triple bonds with other electronegative iotas like oxygen and nitrogen.
- These two carbon properties, i.e., catenation and numerous bond development, have several allotropic structures.
Existence of Carbon Compounds
Carbon is one of the more broad, weighty components – it might make up practically 0.5 per cent of the universe's mass. The nearby planetary group was shaped from a material that was very wealthy in carbon. Moreover, after all, the component makes up 0.025 per cent of Earth's covering, and a large portion of this carbon is bound up in rocks and minerals, such as limestone and chalk. Be that as it may, carbon is profoundly packed in living animals and records for almost one-fourth of iotas in our tissues.
Carbon exists mainly in three forms:
Straight Chains:
One carbon iota is attached to another carbon framing a straight line in such a plan without building up any branches. Low sub-atomic weight hydrocarbons exist in linear chains. For instance ethane.
Branches:
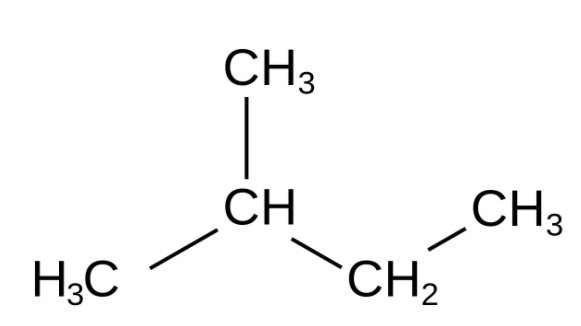
Carbon mixes with higher sub-atomic weight generally exist in expanded structures; for example, one of the carbon iotas is attached to more than two carbon particles—for instance, isopentane.
Rings
In this sort of game plan, at least three carbon particles are connected to structure shut cycles. Such mixes are otherwise called cyclic mixes—for instance, cyclohexane.
In Class 12: In chapter Aldehydes, Ketones and Carboxylic Acids, carbon compounds have been explained in detail with different carbon elements with their properties, questions, illustrations, and reactions also. The chapter has a weightage of 28 Marks.
Illustrated Examples
- Identify the functional group as below:
Halo Group of Carbon Compound
- If the molecule formula of a compound is C26H52, then what type of compound is this?
It is an unsaturated hydrocarbon
- Alcohol beverages contain which chemical compound in it?
Ethyl Alcohol
Explore exams which ask questions on Carbon and its Compounds
Select your preferred stream
FAQs on Carbon Compounds
Q. For what reason is it essential to include sp2 hybridisation to clarify holding in C2H4?
Q. What will be the next carbon compound of CH3Cl?
Q. Ethanol, ethanoic acid, and propanal are some carbon compounds; which compound is related to each other?
Q. Which is the second member present in the series of the alkane?
News & Updates
Carbon and its Compounds Exam
Student Forum
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test