Carbohydrates
Carbohydrates are biological molecules made up of Carbon, Hydrogen, and Oxygen. The general formula of carbohydrates is Cx(H2O)y. They are also known as saccharides. Based on their hydrolysis behaviour, they divided into three groups: Monosaccharides, Oligosaccharides, Polysaccharides.
Based on the number of carbon atoms and the functional group present, monosaccharides are divided into aldoses and ketoses. So, here Glucose and Fructose come under the category of Monosaccharides.
Glucose
Glucose is a six-carbon sugar that occurs freely and also in combined form in nature. Glucose is available in regular foods like sweet fruits, honey, starch, table sugar. Glucose acts as building blocks for cellular structures and energy sources for muscles and the brain during physical activity. Glucose is aldohexose because of having aldehyde as a functional group.
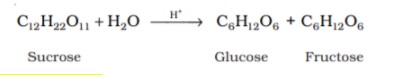
Preparation of Glucose is done in the laboratory either by Sucrose(Cane Sugar) or Starch.
The reaction below is the hydrolysis of the disaccharide, Sucrose to obtain glucose and fructose in equal amounts.
Fructose
Fructose is a ketohexose due to ketone as a functional group at carbon number 2. Generally found in fruits, honey and vegetables. Fructose used as a sweetener for low-calorie foods like carbonated sodas, cereals and yoghurt. Apart from that, it enhances glucose metabolism.
Difference between Glucose and Fructose in Tabular column
| GLUCOSE |
FRUCTOSE |
|---|---|
| Glucose is a six-membered ring. |
Fructose is a five-membered ring. |
| Glucose has an aldehyde functional group - Aldohexose. |
Fructose has a ketone functional group - Ketohexose. |
| It forms a pyranose ring structure. |
It forms a furan ring structure. |
| It releases energy fast. |
It releases energy slower. |
| Glucose is known as Grape Sugar, Dextrose. |
Fructose is known as Fruit Sugar, Levulose. |
| Glucose is produced by the enzymatic hydrolysis of sucrose and starch. |
Fructose is commercially manufactured from sugarcane, corn. |
| Glucose is fatless. |
Fructose has fatty substances. |
| Source: Starch and Table sugar |
Source: Fruits and Vegetables, Sugar Cane |
| It is used for vitamin A production, starch and glycogen. |
It is used to produce ATP and to build glycogen. |
Biomolecules in Class 12
In this chapter of biomolecules, the subtopics are carbohydrates and its types like mono, oligo and polysaccharides. Further, you can learn about their characteristics and applications in detail. This chapter introduces you to topics like Nucleic acids and proteins.
Illustrated Examples from NCERT
1. Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose?
Answer: Monosaccharides - Galactose, Fructose, Ribose, 2- deoxyribose
Disaccharides- Maltose, Lactose.
2. Explain why Glucose and Sucrose are soluble in water in comparison to cyclohexane or benzene.
Answer: Both Sucrose and Glucose have OH groups that form intermolecular H-bonds with water. So, because of Hydrogen bonding, they are soluble in water. Cyclohexane or benzene are insoluble because of the absence of Hydrogen bonds.
3. How can you tell the difference between glucose and fructose in a chemical test?
Answer: Seliwanoff's test determines aldose and ketose sugars. When added to the solution, the red colour is formed and pink in colour when added to the aldoses.
[Image courtesy: NCERT]
FAQs on Glucose and Fructose
Q: What are the functions of carbohydrates?
Q: What is the difference between monomer and monosaccharide?
Q: What is the Glycosidic Linkage?
Q: What are Reducing Sugars?
Q: What are some of the physical properties of Glucose and Fructose?
News & Updates
Biomolecules Exam
Student Forum
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test