Introduction
Monosaccharides, usually with an unbranched C-chain, are poly-hydroxy-aldehydes or -ketones. The organic compound with the formula (CH2O), with n > 3, is a carbohydrate.
What do monosaccharides mean?
The most basic carbohydrate form is monosaccharides. Most species create and store energy by breaking down glucose monosaccharides and harvesting the released energy. In terms of the number of carbon atoms and even the functional group attached to it, this kind of glucose is categorised. The aldehyde-derived monosaccharide is known as aldose, and those containing the ketone group are known as ketose.
Monosaccharide Structure
When we talk about the structure of monosaccharides, the formula for all monosaccharides is (CH2O) n. Here, the two atoms of hydrogen and one atom of oxygen are bound to the core carbon molecule. As oxygen bonds with hydrogen, a hydroxyl group is formed. Since 4 bonds can form on carbon, multiple carbon molecules bond together.
A double bond with oxygen in the chain, which is called a carbonyl group, is formed by one of the carbons. If it is formed at the end of the chain, based on its location, then the monosaccharides are known to be a part of the aldose family, and if they are formed in the centre of the chain, then they belong to the ketose family.
Monosaccharides Examples
Here are a few examples of a monosaccharide:
- Glucose
In the course of cellular respiration, glucose molecules can be broken down by glycolysis. Glucose may be joined together to form polysaccharides in long strings of monosaccharides. This stuff is produced as cellulose in plants. In plants, cellulose covers each cell, allowing plants to stand erect and turgid.
- Fructose
Fructose belongs to a category called ketose. The same enzyme is broken down by variously formed monosaccharides. Oligosaccharides are formed when fructose is interacting with other monosaccharides. With the aid of a glycosidic bond, Sucrose contains a fructose molecule adjoining a glucose molecule.
- Galactose
In the form of milk, galactose is formed by mammals. Lactose retains a lot of energy in its bonds, and particular enzymes are formed by mammalian springs to break down the bonds apart.
Monosaccharides in Class 12
This concept is taught in chapter Biomolecules. In this, you will learn about the types, examples, and structure of monosaccharides. The weightage of this chapter is 5 marks in the final exam.
Illustrated Examples
1. How are monosaccharides formed?
Answer: The dehydration reaction bonds the two monosaccharides leading to the loss of a water molecule and the forming of a glycosidic bond (also called a condensation reaction or dehydration synthesis). The glycosidic bond on the product monosaccharide is possible to be formed between any kind of hydroxyl group.
2. Is carbohydrate a monosaccharide?
Answer: The monosaccharide and the disaccharide groups comprise basic carbohydrates.
3. Where are monosaccharides found?
Answer: Typically, monosaccharides are found in the cytosol (cell sap). In certain fruits and vegetables, such as corn, peas, and sweet potatoes, their content is very high.
FAQs on Monosaccharides
Q: What are monosaccharides?
A: Monosaccharides are the only simplest units of carbohydrates and they are the simplest form of sugar. In other words, It is the most basic form of carbohydrates. They are made up of hydrogen, carbon and oxygen atoms. They are the building blocks of more complex carbohydrates i.e., they can join together and form complex carbohydrates.
For example:
- Monosaccharides form
- 3-10 of them form
- 11 or more of them form
Monosaccharides are used to produce and store energy. Most organisms create energy by breaking down the monosaccharide glucose and harvesting the energy released from the bonds. Other Monosaccharides are used to form long fibers, which can be used as a form of cellular structure.
Examples of Monosaccharides are glucose, fructose, and galactose.

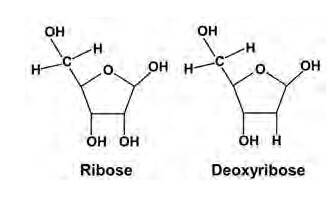
Q: Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
A: (i) 1.Ribose, 2-deoxyribose, galactose, and fructose are
Monosaccharides are the simplest units of carbohydrates which cannot be hydrolyzed into simpler compounds.
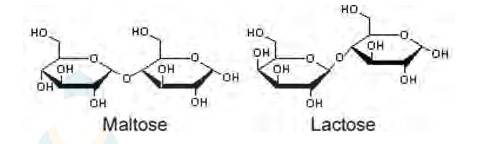
(ii) Maltose and lactose are
A disaccharide is a carbohydrate that is formed when two monosaccharides are joined together and a molecule of water is removed from the structure. Lactose is a disaccharide formed from the combination of galactose and glucose.
Q: What are reducing sugars?
A: All those carbohydrates which reduce Fehling’s solution to red precipitate of Cu2O or Tollen’s reagent to metallic Ag is called reducing sugars. All monosaccharides (both aldoses and ketoses) and disaccharides except Sucrose are reducing sugars.
Q: Glucose or sucrose is soluble in water but cyclohexane or benzene (simple six membered ring compounds) is insoluble in water. Explain.
A: Glucose and sucrose are carbohydrates (optically active polyhydroxy aldehydes or ketones).
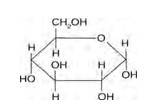
Structure of glucose:
Structure of sucrose:
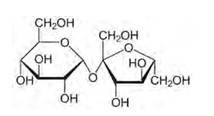
As you can see both the compounds have five –OH and eight –OH groups respectively. These –OH groups are responsible for the extensive hydrogen bonding with water. This –H bonding is responsible for the solubility of glucose and sucrose in water.
In case of cyclohexane or benzene (simple six-membered ring compounds) , they do not contain any – OH groups. Hence, they cannot undergo –H bonding with water and are insoluble.
Q: The melting points and solubility in water of amino acids are generally higher than that of the corresponding halo acids. Explain.
A:
A 14.4 Amino acids are organic compounds containing amine (basic) and carboxyl (acidic) functional group with a specific side chain. Both acidic and basic group are present in the same molecule. In, aqueous solution carboxyl group can lose a proton (H+) and amino group can accept a proton (H+) giving rise to the dipolar ion called as zwitter ion. Zwitter ion is shown below:
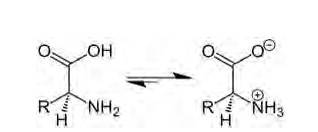
In this zwitter ion there is the presence of both positive as well as negative charge, so there is the development of strong electrostatic force of attraction between the molecules and the water. For this reason solubility of amino acids is higher. Due to strong electrostatic interaction there is formation of strong bond between them, hence the melting point of amino acid is higher than the halo acids (as they do not exhibit such dipolar behaviour).
Q: Which are the 3 monosaccharides?
A: The three monosaccharides which are essential in nutrition are glucose, fructose, and galactose.
Q: Which monosaccharide is the simplest?
A: Two three-carbon trioses are the simplest monosaccharides: glyceraldehyde, an aldose, and dihydroxyacetone, a ketose.
Q: Which monosaccharide is the sweetest?
A: Fructose is a basic ketonic monosaccharide, or fruit sugar, and the sweetest sugar present in many plants where disaccharide sucrose is also bound to glucose to form.
Q: Is orange juice a monosaccharide?
A: Although most beverages that are commonly sold in supermarkets contain abnormally elevated sugar content, orange juice generally does not. Orange juice is made of other potentially beneficial ingredients and a mixture of fructose, glucose, and sucrose is the sugar found in orange juice.
Q: Is rice a monosaccharide component?
A: There is only one subunit of sugar in the monosaccharides, and they constitute the essential units of all carbohydrates. Hence it is a monosaccharide.
News & Updates
Biomolecules Exam
Student Forum
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test
