Dehydrogenation aka the process of dehydration of alcohol
When alcohol and protic acids react with each other, it loses an ion of water and form alkenes. The reaction that took place is known as Dehydration of Alcohol or Dehydrogenation. It is an elimination reaction and can be classified into primary, secondary, and tertiary alcohols.
The reaction of alcohol Dehydration
When dehydration of alcohol is formed, the following reaction takes place:
C2H5OH → C2H4 + H2O
Alcohol dehydration is a process of elimination, inverted to the addition and substitution reaction.
Process of Dehydration of Alcohols
There are two types of mechanism, E1, and E2. The primary alcohols use the E2 elimination reaction mechanism, whereas tertiary and secondary alcohols follow the E1 elimination reaction mechanism.
There are three steps in total for the mechanism of dehydration of alcohols, which are explained below:
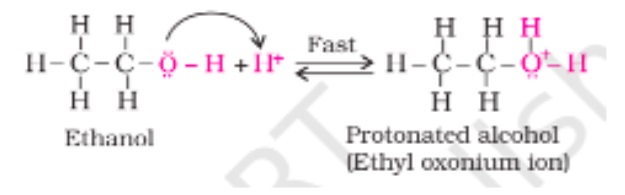
- Protonation Alcohol’s Creation:
The protic acid acts with alcohol in this step. In this, the isolated pairs are present in the oxygen molecules, and it serves as a Lewis base. The reversible action of protonation of alcoholic oxygen occurs, and it creates a leaving group that takes place quickly.
- Carbocation Creation:
In this step of dehydration of an alcohol, carbocation forms by breaking the bond of C-O. This is considered one of the slowest steps in the process of dehydration of alcohols. Hence, it affects the creation of the carbocation rate.
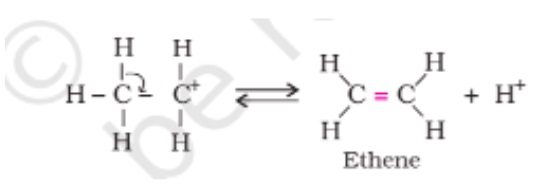
- Creation of Alkene:
The last step in the process of dehydration of alcohols is the creation of alkene. In this step, with the help of the base, the protons are being removed. Carbocation next to the carbon atom breaks the existing C-H bond and form C=C and alkene is formed at the end.
Dehydration of alcohols in chapter 12
For the current year of 2021, the chapter on alcohols, phenols, and ethers holds a weightage of 6 marks including one short question (3 marks), one very short question (2 marks), and one objective type question (1 mark).
Illustrated Examples
Example 1) – Illustrate the mechanism of dehydration of alcohol in three points up until the point Ethene is formed.
Answer - The following steps clearly depicts the role of the mechanism and how it plays a crucial part in the dehydration process –
Step 1 – this steps how the protonated alcohol is formed
Step 3 - How Ethene is formed after losing a proton:
Example 2) – Explain the sequential relative case order, which is followed by the process of alcohol dehydration.
Answer - the dehydration process depicts more of a step by step lead to the primary formation of ether which can be understood from a hierarchy as follows:
Tertiary > Secondary > Primary
Example 3) – When alcohol cannot be dehydrated?
Answer – the alcohol cannot be dehydrated easily because the reaction is not heated up sufficiently to dehydrate.
A. Dehydrogenation eliminates one or more hydrogen molecules, whereas, in dehydration, one or more water molecules are eliminated or released.
A. Alkenes are formed with the elimination reactions. The creation of alkenes consists of alkane alkaline dehalogenation, alcohol oxidation, etc.
A. The reaction between hydrogen ions and other compounds is simply known as hydrogenation.
A. The tertiary alcohols dehydrates the fastest as compared to primary and secondary alcohols due to the carbonation process.
A. The secondary alcohols can be identified by the colour change when reacts with the potassium dichromate compound. After the reaction, the colour of the secondary alcohols changes into multiple shades, making it easier to determine the reason.
FAQs regarding Dehydration of Alcohols
Q. Explain the difference between dehydrogenation and dehydration?
Q. How alkenes are formed?
Q. What is hydrogenation?
Q. Which alcohols are dehydrated the fastest?
Q. How secondary alcohols can be identified?
News & Updates
Alcohols, Phenols and Ethers Exam
Student Forum
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test