Introduction
Thermodynamics is the study of energy transformation. It is based on the first and last states of the changing system. Laws of thermodynamics suit a universe in equilibrium or a system transiting from one equilibrium state to another.
Third law of thermodynamics
The third law of thermodynamics states that the entropy (S) or chaos of a system approximates a constant value if the temperature of that system reaches near absolute zero.
At absolute zero temperature, the system must have the lowest possible energy. Such a state with the least possible energy in a system is called the constant system.
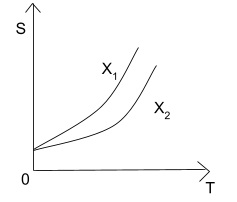
Here the two systems X1 and X2 are represented on an entropy- temperature graph.
As the system approaches near absolute zero, the number of microstates gradually decreases. The only attainable microstate at absolute zero is the ground state.
Entropy measures the spontaneity of any system. Increased movement in a system means that the randomness is higher. The entropy S of a system is given as-
S- S0= kB ln
Where S0 is the initial entropy, kB is the Boltzmann constant= 1.38064852 × 10-23 m2 kg s-2 K-1 and is the number of microstates.
If the atoms move freely and are randomly distributed in the gaseous state, it indicates high entropy. In the solid-state, the atoms are packed together and thus have less space to move freely. In this case, the entropy is low. So, the order of entropy for states is given as- Gaseous> Liquid> Solid.
Spontaneity in term of ( ∆S )-
∆S(total) = ∆S(universe) = ∆S(system) + ∆S(surrounding) .
About this topic in class 11
The topic- third law of thermodynamics is a part of the chapter- thermodynamics in the syllabus of class 11. This chapter carries a weightage of around 7-8 marks in the examination.
system will never decrease if it is in a spontaneous process.
Illustrated examples
1. For oxidation of iron, 4Fe (s)+ 3O2 (g) → 2Fe2O3 (s) entropy change is – 549.4 JK-1mol-1 at 298 K. Despite the negative entropy change of this reaction, why is the reaction spontaneous? (∆rH 0 for this reaction is –1648 × 103 J mol –1)
Solution:
One decides the spontaneity of a reaction by considering ∆S total (∆Ssys+ ∆S Ssurr).
To calculate ∆Ssurr, we consider the heat absorbed by the surroundings, which is equal to – ∆rH. At temperature T, entropy change of the surroundings is-
( ∆Ssurr )= – ∆rH/T (at constant pressure).
∆ ∆ = −1648x 103 J mol-1/ 298 K = 5530 JK-1 mol-1.
Thus, total entropy change for this reaction-
∆rStotal = (5530 + (-549.4))JK-1mol-1= 4980 JK-1mol-1.
So, the reaction is spontaneous.
2. Calculate the entropy change when 3.6g of liquid water is completely converted into vapour at 100°C. The molar heat of vaporization is 40.85.
Solution:
Entropy change for 3.6g water =6.083JK-1g-1×3.6g
=21.89JK-1
3. The latent heat of vaporisation of water is 540cal g-1 at 100°C. Calculate the entropy change when 1000 g water is converted to steam at 100°C.
Solution:
ΔSvap=ΔHvap/T= (540×1000)/373= 1447cal
Frequently asked questions
Q: Who developed the third law of thermodynamics?
Q: What is entropy?
Q: Why is the temperature of zero Kelvin non-attainable?
Q: What does the first law of thermodynamics state?
Q: What does the second law of thermodynamics state?
News & Updates
Thermodynamics Exam
Student Forum
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test