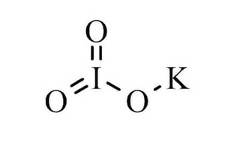What is potassium iodate?
Potassium iodate is primarily a very reactive oxidizing agent that can cause a fire when brought in contact with any combustible or inflammable material. The reaction is highly exothermic in nature. Any reducing agent can also cause the same effect when treated in the presence of potassium iodate. Potassium iodate comprises K+ ions and IO3- ions in the ratio 1:1. The concentration of both ions is in an equal proportion.
Structure of potassium iodate
Iodine has two forms of salt; one of them is iodide, and the other is iodate, which is potassium salt. The potassium salt is less soluble and more stable as compared to iodide because of its moist conditions. However, both of them are considered as iodised salt. Potassium iodate is formed by reacting with potassium hydroxide or when iodine reacts with potassium hydroxide, the second reaction, however, forms iodide and iodate.
Uses of potassium iodate
KIO3 is the chemical formula for potassium iodate. It can be produced by reacting an iodic base with a potassium base. One of the primary uses for potassium iodate is the iodisation of common salts. Potassium iodate is a primary source of chemical compounds used in several other applications as well, iodisation of common salts being one of the commercial applications. Iodometric work becomes very convenient when performed using potassium iodate.
Physical properties
Here are some properties of potassium iodate:
- The chemical formula is KIO3
- Its density is 3.89 g/cm3
- Its molecular weight is 214.001 g/mol
- The melting point is 560°C
Properties of Potassium Iodate
Following are some chemical properties of potassium iodate -
Potassium iodate in the presence of a strong acid, such as sulphuric acid, results in the production of potassium sulphate, water, and iodine. Here is the process:
Uses of Potassium Iodate
Some other uses of potassium iodate that indicate the compound’s versatility are as follows
- Potassium iodate is also used for domestic purposes. It’s used as a food maturing agent and as a conditioner for dough.
- Perhaps one of the most interesting uses of potassium iodate is in light polarisation filters as an electronic chemical.
- It is used in the manufacturing of tablets that help to regulate the thyroid gland.
- Potassium iodate is also used for therapeutic purposes and as an antiseptic.
Potassium Iodate in Class 10
For the current year of 2021, the chapter ‘Acids, Bases and Salts’ holds a weight age of 4 marks, which includes 2 short questions; 2 marks each, including a question on potassium iodate for students of Class 10.
Illustrated Examples
1. The following reaction takes place between potassium iodate (KIO3) and sodium sulphite (Na2SO3):
2. The following table outlines the apparatus used in the above reaction.
3. After carefully following the procedure, the following table should be used to input the findings and the final results.
FAQs on Potassium Iodate
Q: Can potassium iodate be labelled as an acid or a base?
Q: What’s the reason behind using potassium iodate in salt?
Q: What is the chemical formula for potassium iodate?
Q: What is the molecular weight for KIO3?
Q: Name one domestic use of KIO3.
News & Updates
Acids, Bases and Salts Exam
Student Forum
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test