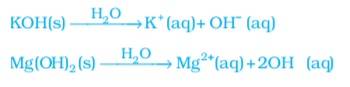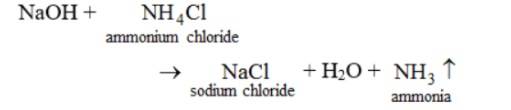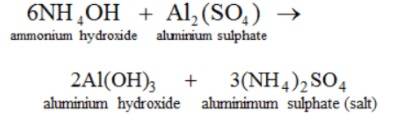A base is a chemical species that donates electrons, accepts protons or releases hydroxide ions(OH-) ions in aqueous solution. The classification of bases is according to their degree of dissociation in water and reactivity.
Strong base completely dissociates its ions in water - NaOH, KOH.
A weak base is an incomplete dissociation in water - NH3
Superbase is better at deprotonation than the strong base. Examples of superbases are Sodium hydride(NaH), ortho-diethynylbenzene dianion.
Properties of Bases
- The bases have pH value in the range of 7-14.
- They are bitter in taste, corrosive.
- Aqueous solutions of bases conduct electricity due to the formation of ions.
- The bases are slippery and soapy to touch.
- Never taste or touch them as they may cause harm.
Reaction with Metals
Hydrogen gas is released when a base like NaOH reacts with Zinc metal. However, it is not possible with all metals.
2NaOH + Zn → Na2ZnO2 + H2O
Reaction with Acid
The reaction between an acid and base to give salt and water is known as neutralization reaction.
NaOH + HCl → NaCl + H2O
Reaction with Water
The bases which are soluble in water are called alkalis. They generate Hydroxide ions in water. In general, the mixing of a base with water results in the decrease of OH- ions per unit volume.
Reaction with Oxides
Bases react with acidic oxides to produce salts and water. Acidic oxides are oxides of nonmetals or metals of high oxidation states.
Reaction with an Ammonium Salt
On reaction with ammonium salts, ammonia gas is released. For example, the reaction of NaOH with ammonium chloride yields ammonia, sodium chloride and water.
Reaction with Other Salts
Bases undergo reaction with other salts to produce salts and other bases.
Here, ammonium hydroxide reacts with a solution of ammonium sulphate to yield aluminium hydroxide and ammonium sulphate.
Properties of Bases for Class 10
In this chapter, you will study the reactions of acids and bases with the other chemicals. The weightage of this topic is less than 5 marks. Questions are in the form of both objective and subjective type.
Illustrative Examples
- What is the pH of 0.05M solution of KOH?
KOH is a strong base and dissociate completely into K+ and OH-
pOH = - log (0.05)
pOH = -(-1.3)
pOH = 1.3
pH = 14- pOH = 14-1.3 =12.7
- Calculate the pH of 1 × 10–4 molar solution of NaOH?
NaOH(aq) ⎯→ Na+(aq) + OH–(aq)
One mole of NaOH would give one mole of OH– ions.
[OH–] = 1 × 10–4 mol L–1
pOH = –log[OH–] = –log × 10–4 = –(–4) = 4
Since pH + pOH = 14
pH = 14 – pOH = 14 – 4 = 10
- What is meant by Lewis acids and Lewis bases?
Lewis acid accepts an electron pair (electrophile) and has vacant orbitals.
Lewis base donates an electron pair (nucleophile) and has lone-pair electrons.
FAQs on Properties of Bases
Q. What are the common uses of bases?
- As a reagent in the laboratory.
- In the preparation of medications.
Q. What are the differences between alkalis and bases?
Q. What are Antacids?
Q. Name list of common indicators in basic solution?
Q. Why is Sodium hydroxide called caustic soda?
News & Updates
Acids, Bases and Salts Exam
Student Forum
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test