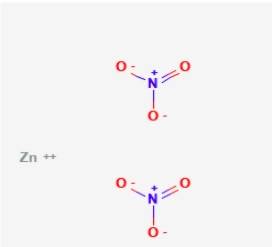
What is Zinc Nitrate?
Zinc Nitrate is a colourless inorganic compound which is non-combustible, but it speeds up the burning of combustible products. The chemical formula of Zinc Nitrate is Zn(NO3)2.
Physical properties of Zinc Nitrate
Let us now look at the physical properties of Zinc Nitrate.
- Anhydrous Zinc Nitrate’s molecular weight is 189.4 g/mol, and hexahydrate Zinc Nitrate is 297.49 g/mol.
- It is a colourless, odourless and crystalline solid.
- Zinc Nitrate is highly soluble in water and alcohol.
- It is non-combustible but can act as a driver is speeding up the burning in combustible products.
- The topological polar surface of Zinc Nitrate is 126 Ų.
- The density of hexahydrate Zinc Nitrate is 2.065 g/cm3.
- The melting point of anhydrous Zinc Nitrate is 110 0C, trihydrate Zinc Nitrate is 45.5 0C, and hexahydrate Zinc Nitrate is 36.4 0C.
- The hexahydrate Zinc Nitrate decomposes at 125 0C.
- The magnetic susceptibility of Zinc Nitrate is -63.0.10-6 cm3/mol.
Chemical properties of Zinc Nitrate
Let us look at some chemical properties.
1. When Zinc Nitrate is heated, it decomposes and releases toxic Zinc Oxide. Proper care has to be taken while handling it.
Zn(NO3)2 — 2ZnO + 4NO2 + O2
2. When Zinc is chemically reacted with Nitric Acid, it has two types of reactions depending upon the acid’s concentration.
a. When reacted with dilute Nitric Acid
Zn + 2HNO3 — Zn(NO3)2 + H2
b. When reacted with concentrated Nitric Acid, along with Zinc nitrate, it produces ammonium nitrate which is used as an agricultural fertiliser.
Zn + 10HNO3 — 4Zn(NO3)2 + 3H2O + NH4NO3
Uses of Zinc Nitrate
Let us look at some of the uses of Zinc Nitrate.
- Zinc Nitrate is used as a liquid fertiliser.
- It is used in the manufacturing of medicines.
- It is used as a strong oxidising agent.
- It is used as a dyeing agent in the textile industry.
- Zinc Nitrate acts as a catalyst during the manufacturing of resin.
- Zinc Nitrate is used in the synthesis of polymers.
- Zinc Nitrate is used in the manufacture of plastic cutlery and other plastic items.
- It is used in the manufacture of different compounds for different laboratories.
- Zinc Nitrate is used in the manufacturing of pesticides.
Zinc Nitrate in Class X
Zinc Nitrate and its other compounds have been explained in the chapter ‘Acids, Bases, and Salts’. The chapter covers different reactions with different compounds and how to identify between the acids and bases. It also talks about the reaction between an acid and base.
Illustrative Examples
1. What happens when iron filings are added to dilute hydrochloric acid?
Answer: When iron filings are added to dilute HCl, iron chloride, and hydrogen gas are produced.
2. Identify the incorrect statement
2PbO + C — 2Pb + CO2
a. CO2 is getting oxidised.
b. Carbon is getting oxidised.
c. PbO is getting reduced.
d. Lead is getting reduced.
Answer: (d) and (a)
3. What kind of reaction is the below-mentioned equation?
Fe2O3 + 2Al — Al2O3 + 2Fe
a. Double displacement reaction
b. Displacement reaction
c. Decomposition reaction
d. Combination reaction
Answer: (b)
FAQs on Zinc Nitrate
Q: What is the chemical formula of Zinc Nitrate?
Q: What is the molecular weight of Zinc Nitrate?
Q: What is the melting point of Zinc Nitrate?
Q: What is the density of Zinc Nitrate?
Q: How does Zinc Nitrate help in the manufacture of fertilisers?
News & Updates
Acids, Bases and Salts Exam
Student Forum
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test