
Salviya AntonySenior Executive - Content
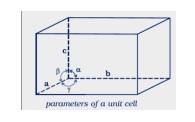
Dimension of a unit cell: Unit cell is the smallest portion of a crystal lattice which, when repeated in different directions, generates the entire lattice. The arrangement of particles in a crystal lattice can be described by specifying the dimension of the unit cell. A unit cell is characterised by: (i) its dimensions along the three edges, a, b and c. These edges may or may not be mutually perpendicular. (ii) angles between the edges, α (between b and c) β (between a and c) and γ (between a and b). Thus, a unit cell is characterised by six parameters, a, b, c, α, β and γ. A unit cell can be defined as the smallest repeating structural unit of a crystal lattice.
Unit Cell: Categories
Unit cells are divided into two categories, namely primitive and centred unit cells.
Primitive Unit Cells: When constituent particles are present only on the corner positions of a unit cell, it is called a primitive unit cell.
Centred Unit Cells: When a unit cell contains one or more constituent particles present at positions other than corners in addition to those at corners, it is called a centred unit cell.
The Centred unit cells are of three types:
Body-Centred Unit Cells: This unit cell contains one constituent particle (atom, molecule or ion) at its body-centre besides the ones that are at its corners.
Face-Centred Unit Cells: This unit cell contains one constituent particle present at the centre of each face, besides the ones that are at its corners.
End-Centred Unit Cells: In such a unit cell, one constituent particle is present at the centre of any two opposite faces besides the ones present at its corners.
Here's a brief note on the topic of "Dimensions of a Unit Cell": In the study of crystalline solids, a fundamental concept is the "unit cell." A unit cell can be defined as the smallest repeating structural unit of a crystal lattice. The arrangement of particles in a crystal lattice can be described by specifying the dimensions of the unit cell.
Unit Cell Parameters
- To fully describe the dimension of a unit cell, three parameters are commonly used: a, b, and c. These parameters represent the lengths of the unit cell edges along the three perpendicular axes, typically referred to as the x, y, and z axes.
- The angles between these edges are denoted as α, β, and γ.
- Therefore, the dimensions of a unit cell are determined by the lengths a, b, and c, as well as the angles α, β, and γ between them.
Types of Unit Cells:
There are several types of unit cells based on their dimensions and angles:
Cubic Unit Cell: In a cubic unit cell, all the edges are of equal length (a = b = c) and all angles are 90 degrees (α = β = γ = 90°).
Tetragonal Unit Cell: In a tetragonal unit cell, two edges (a and b) have the same length (a = b), while the third edge (c) is of a different length, and all angles are 90 degrees.
Orthorhombic Unit Cell: In an orthorhombic unit cell, all three edges (a, b, and c) have different lengths, and all angles are 90 degrees.
Rhombic Unit Cell: In a rhombic unit cell, all three edges (a, b, and c) have different lengths, and all angles are not equal (α ≠ β ≠ γ).
Monoclinic Unit Cell: In a monoclinic unit cell, all three edges (a, b, and c) have different lengths, and only one angle (β) is not equal to 90 degrees.
Triclinic Unit Cell: In a triclinic unit cell, all three edges (a, b, and c) have different lengths, and all three angles (α, β, and γ) are not equal and not equal to 90 degrees.
Unit cell calculation
We can find the volume of the unit cell using the geometry and attributes of unit cells. Using volume and mass of atoms, we can calculate the density of the unit cell. A crystal lattice is represented in terms of unit cells. we can evaluate the density of crystal lattices by evaluating the density of unit cells.
Density of unit cell = mass of unit cell/volume of unit cell
Unit Cell: Significance
The knowledge of unit cell parameters is important for determining the density of a crystal, understanding its symmetry, and predicting its physical properties.These parameters help in identifying and classifying different crystal systems, which are important in various fields, including materials science, mineralogy, and solid-state chemistry.
In conclusion, understanding the dimensions of a unit cell is fundamental when studying crystalline solids. These parameters provide essential information about the internal structure of crystals and are instrumental in unraveling their properties and behavior. In Class 12 NCERT Chemistry, the topic Dimensions of a Unit Cell is an important concept within the study of solid state. This topic is particularly relevant when discussing the arrangement of particles in crystalline solids, which are characterised by their highly ordered and repeating three-dimensional structures.
FAQs on Unit Cell
Q: Refractive index of a solid is observed to have the same value along all directions. Comment on the nature of this solid. Would it show cleavage property?
A: As isotropic solid has the same value of physical properties when measured along different directions. Therefore, the given solid, having the same value of refractive index along all directions, is isotropic in nature. Hence, the solid is and amorphous solid. When an amorphous solid is cut with a sharp edged tool, it cuts into two pieces with irregular surfaces
Q: Solid A is a very hard electrical insulator in solid as well as in molten state and melts at extremely high temperature. What type of solid is it?
A: The given properties are the resource of a covalent or network solid. Therefore, the given solid is a covalent or network solid. Examples of such solid are quartz (SiO) and diamond(C).
Q: Name the parameters that characterize a unit cell.
A:
The six parameters that characterize a unit cell are as follows.
(i) Its dimensions along the three edges, a, b, and c these edges may or may not be equal.
(ii) Angles between the edges these are the angles (between edges b and c), (between edges a and c), and (between edges a and b)
Q: Explain how vacancies are introduced in an ionic solid when a cation of higher valence is added as an impurity in it.
A: Two or more cations of lower valency are replaced by a cation of higher valency to maintain electrical neutrality. Hence some cation vacancies are created. For example : In an ionic solid 'NaCl' , is impurity of Sr2+ is added (as SrCl2) then two Na+ ions left thus lattice sited. To maintain electrical neutrality one lattice site is occupied by Sr2+ ion while other lattice site will remain vacant.
Q: What type of substances would make better permanent magnets, ferromagnetic or ferrimagnetic. Justify your answer.
A: Ferromagnetic substances would make better permanent magnets because when the ferromagnetic substance is placed in a magnetic field, all domains get oriented in the direction of magnetic field and strong a magnetic effect is produced.
News & Updates
Atoms and Molecules Exam
Student Forum
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test