Introduction
Chemistry is all about atoms and electrons that have either gained or lost their weight by eliminating or adding one or two valence electrons, carrying either positive or negative charge. The ions with a negative charge are called anions, and cations are those with a positive charge. As both have opposite charges, they are drawn to each other and form an ionic bond.
Differences between Anions and Cations
| Anions |
Cations |
|---|---|
| 1. Ions that are charged negatively are known as anions. |
1. Positively charged ions are known as cations. |
| 2. Anions are formed from an atom acquiring an electron. |
2. Cations are formed by an atom's loss of electrons. |
| 3. The number of e- in anions is greater than the number of protons. |
3. The number of e- in a cation is smaller than the number of protons. |
| 4. Type of element: Nonmetal |
4. Type of element: Metal |
| 5. Type of electrode used: Anode |
5. Type of electrode used: Cathode |
| 6. Anions combine the ionic bonds with the cations to form them. |
6. To form the ionic bonds, cations mix with the anions. |
| 7. Eg: Fluoride (F-), Bromide (Br-), Iodide (I-), Nitride (N3-) |
7. Eg: Iron (Fe2+), Sodium (Na+), Lead (Pb2+). |
Cation and Anions in Class 10
Cations and anions are covered in almost every chapter of chemistry, as reactions are presented in most chapters. There is a chapter titled ‘Atoms and Molecules’ that goes into detailed study.
Cation and Anions in Class 12
In class 12, you have to have in-depth knowledge of cations and anions for understanding the detailed concepts of any chapter. The entire organic chemistry is based on reactions, and no reaction can be solved without knowing the charge on a cation or an anion.
Illustrated Examples
1. How are anions and cations formed?
A: As a metal loses electrons, cations (positively-charged ions) and anions (negatively-charged ions) are formed, and a nonmetal gains those electrons.
2. What are cations and anions supposed to mean?
A: Due to the cumulative number of e- being unequal to the aggregate number of protons, an ion's net charge is non-zero. With fewer electrons than protons, a cation is a positively charged ion, while an anion is negatively charged, with more e- than protons.
3. What are the two ion types?
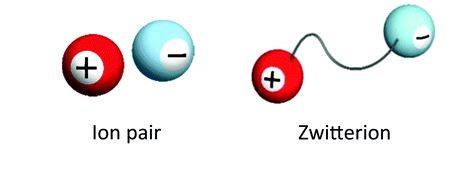
There are more electrons than protons in anions, and they thus have a net negative charge. There are more protons in cations than electrons, so they have a net positive charge. In different positions throughout a molecule, ‘zwitterions’ are neutral and contain both positive and negative charges.
FAQs on Difference between Anions and Cations
Q: What is the difference between a cation and an anion?
Q: Is a cation negative or positive?
Q: What is a Cation Test?
Q: Is al3+ a cation or anion?
Q: Is an anion negative or positive?
News & Updates
Atoms and Molecules Exam
Student Forum
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test