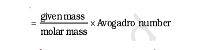What is the molar mass?
An atom or a molecule, both are small in size. But we also know that everything that we see is made of matter and contains the atom. So, there has to be a particular weight of these molecules and atoms as well. One mole of the sample's weight is called molar mass. The connection of atomic masses is used to calculate the weight of the molar mass. The sample of a chemical compound is when divided by the amount of the substance, the molar mass is calculated.
The synonyms of molar mass include molarity and molecular weight or molar weight. This formula is widely used for the calculations of other compounds like ionic salts. The unit of molar mass is 'kg/mol' It is defined in such a way, that the average weight of one mole is equal to the molecular weight of a compound. For example, the molecular weight of water is 18.0153 g/mol, and the average weight of one molecule of water is also 18.0153 daltons.
The calculation of isolated molecules is different. The molecular weight of isolated molecules is calculated by dividing the number of moles.
The formula of molar mass
It is easy to calculate the atomic number and formula masses of atoms and molecules, but they alone cannot be much relevant. They are used to calculate the molar mass of a compound or substance. Chemically, the formula of molar mass is below:
Molar mass = mass/mole = g/mol.
The formula for calculating the number of molecules is as follows:
Image source - ncert
The value of Avogadro’s number is 6.02214154 x 1023 particles per mole. If the value of more moles is needed, multiply the number with Avogadro's number.
The molar mass is used to make the foundation for many other formulas in chemistry. Directly or indirectly, the definition of atomic mass, molar mass, and mole is related. Let us understand that from an example of Carbon-12:
- The mass of one molecule of carbon-12 is 12 gm, and the mass of one atom is also 12 grams.
- If one atom has a molecular mass of 12 grams, it shows that it has 12 atomic mass units.
- The molecular weight or molar mass of carbon-12 is also 12 grams.
Molar class for Class 9
The chapter on atoms and molecules holds a significant weightage of 7 marks per the new pattern 2021. It includes two short questions of three marks each and one objective type question of 1 mark. The question on molar mass can be asked only as a one mark question as it's a simple topic
Illustrated Examples
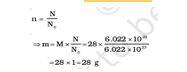
Example 1) – Calculate the mass of 0.5 moles of N2 gas.
Answer – Mass= molar mass x number of moles.
m=M x n = 28 x 0.5 = 14g.
Example 2) – illustrate the calculation of 6.022 x 1023 (Avogadro’s number) number of N2 molecules.
Answer – the illustration of the N2 molecules is as follows:
Image source – ncert
Example 3) – write down the calculations of the following:
- 52 g of He
- 12. 044 x 1023 number of He atoms
Answer – The calculations of the number of moles is below:
class="figure" id=""
FAQs on Molar Mass
Q: What is the difference between mole and molar mass?
Q: How many atoms are equal to one mole?
Q: How many grams does one kilogram have?
Q: What is the unit of molar mass?
Q: How many atoms are present in two moles?
News & Updates
Atoms and Molecules Exam
Student Forum
Popular Courses After 12th
Exams: BHU UET | KUK Entrance Exam | JMI Entrance Exam
Bachelor of Design in Animation (BDes)
Exams: UCEED | NIFT Entrance Exam | NID Entrance Exam
BA LLB (Bachelor of Arts + Bachelor of Laws)
Exams: CLAT | AILET | LSAT India
Bachelor of Journalism & Mass Communication (BJMC)
Exams: LUACMAT | SRMHCAT | GD Goenka Test